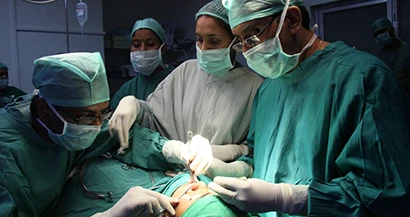
Colorado Springs, Colorado – A team led by Michael Larson, El Pomar Endowed Chair of Engineering and Innovation at the University of Colorado Colorado Springs (UCCS), has developed a laser-based device for closing wounds during nasal surgery that circumvents several technical hurdles.
Now operating as a company, which Larson formed, Tissue Fusion LLC and the CU Office of Technology Transfer recently completed a license agreement that will allow Tissue Fusion to continue developing a new surgical device that utilizes lasers, rather than staples and sutures, to close wounds during nasal surgery.
The two most common nasal surgeries are septoplasty (repair of deviated or deformed septum, thousands performed each year) and rhinoplasty (nose job, more than 150,000 performed each year). Currently, wounds are closed during these surgeries using staples, sutures or intranasal packing, all of which can be dangerous (needles can break, and bleeding can occur) as well as uncomfortable for the patient. After the procedure, techniques like stapling or suturing can cause infection, scarring, or other side effects.
Lasers have been used for decades in place of scalpels to cut tissues in procedures like LASIK eye surgery. Lasers also have the ability to 'weld' tissue together, but have not been widely used in this capacity due to the complexity of the laser (different parameters for different tissues) and the exceptional surgical skill required to use them.
The Tissue Fusion device generates heat and pressure to fuse tissue membranes together, but is designed specifically for use in septoplasty and rhinoplasty, using pre-set parameters to make the device easy to use by a surgeon or a trained medical technician. In addition to making the surgical procedure faster and simpler, the fusion device also has the potential to shorten healing time and reduce side effects like swelling, scarring, and infection.
Tissue Fusion is currently gathering data on the efficacy and safety of the device in controlled trials. The company will use the results in seeking approval from the FDA for clinical use in procedures related to the ear, nose, and throat. Ultimately, the company hopes to introduce additional surgery-specific devices; further research by Larson's team shows that the technology holds promise for spot-welding layers of tissue in a range of surgeries, including microsurgical applications.
"We're pleased to be working in partnership with the University of Colorado to commercialize a new medical technology that is already adding jobs to the Colorado economy," said Larson, who also serves as the company's CEO.
"The technology licensed to Tissue Fusion has been a great example of Colorado's innovation infrastructure, since it represents years of development efforts at MIND Studios in Colorado Springs, as well as a state grant to bring it closer to commercial readiness," said Molly Markley of CU's Technology Transfer Office. "We are looking forward to following the company's progress as it moves towards FDA approval."
Tissue Fusion received a State of Colorado Early-Stage Company grant in 2013 under its Bioscience Discovery and Evaluation Grant program.
Source: UCCS
Latest from Today's Medical Developments
- Children’s National, FDA collaborate to advance pediatric device regulatory tools
- LK Metrology’s eco-friendliness CMMs
- Two patents for microfluidic valves
- AMADA WELD TECH’s blue diode laser technology
- Post-IMTS decline in manufacturing technology orders blunted
- ARS Automation’s FlexiBowl 200
- LMA Consulting urges businesses to restructure supply chains now
- Walter’s WEP01C indexable inserts





