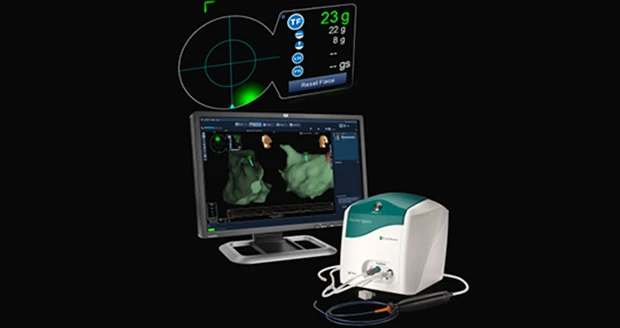
St. Paul, Minnesota – St. Jude Medical Inc. officials announce U.S. Food and Drug Administration (FDA) approval of its TactiCath Quartz irrigated ablation catheter, the company’s newest technology that gives physicians a real-time, objective measure of the force that the catheter applies to a patient’s heart wall during an ablation procedure. TactiCath Quartz contact-force technology was designed to provide physicians with more control to modify that force during ablation procedures in order to create more effective lesions for patients being treated for paroxysmal atrial fibrillation (AF). The technology is associated with a reduction in the rate of AF recurrence when optimal treatment measures are used.
“The number of patients with atrial fibrillation is growing rapidly in the U.S.,” said Dr. Vivek Reddy, director of electrophysiology at Mount Sinai Hospital, N.Y. “As the number of patients impacted by this disease continues to grow, it is important to develop treatment solutions that increase the efficiency and effectiveness of ablation therapies. TactiCath Quartz is an important advancement that provides critical information during ablation procedures.”
The TactiCath Quartz ablation catheter provides electrophysiologists the ability to monitor the amount of pressure that a catheter tip exerts on the endocardium (the layer of tissue that lines the chambers of the heart). Without contact-force sensing technology, physicians have to estimate by touch with their hands the amount of force applied to the heart wall during an ablation. If too little force is applied, effective lesions may not be created and AF may recur, potentially requiring additional treatments. When too much force is applied, there is a risk of tissue injury, which can lead to serious procedure-related complications.
“St. Jude Medical has a long history of introducing leading ablation technologies to the electrophysiology community,” said Eric S. Fain, M.D., group president of St. Jude Medical. “This approval speaks to our commitment to investing in and developing world class, cost-effective solutions that are backed by clinical evidence in order to improve the quality of care for millions of patients impacted by atrial fibrillation.”
Ablation catheters, such as the TactiCath Quartz irrigated ablation catheter, are thin, flexible wires used to help treat irregular heartbeats that impair the heart's ability to effectively pump blood throughout the body. In the U.S., an estimated 2.7 million people are impacted by AF, making the condition the most common type of arrhythmia affecting Americans today.
For more information about AF visit http://health.sjm.com/arrhythmia-answers.
Source: St. Jude Medical
Latest from Today's Medical Developments
- Best of 2024: #1 Article – 2024 Forecast
- Best of 2024: #1 News – 6 trends impact medtech in 2024
- Best of 2024: #2 Article – Cybersecurity in medical devices
- Best of 2024: #2 News – Miniaturized, implantable multi-sensors device to monitor vessels health
- Best of 2024: #3 Article – Intech Athens deploys ZOLLER tool management system to control tooling inventory
- Best of 2024: #3 News – Avation Medical raises $22 million+
- Best of 2024: #4 Article – Sticking to the basics
- Best of 2024: #4 News – Point of care manufacturing