ECA Medical Instruments.
Solvay’s medical-grade Ixef polyarylamide (PARA) was selected by ECA Medical Instruments to manufacture its new TruTORQ and TruPWR family of single-procedure, precision torque limiting instruments for securing medical device implants.
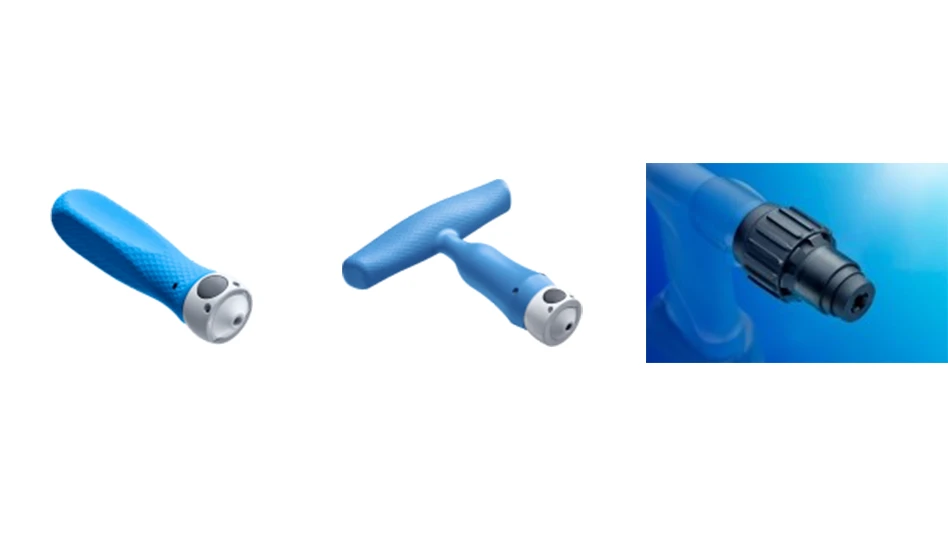
Ixef PARA is ECA Medical Instruments’ choice for the majority of its products, due to the material’s high strength and stiffness, smooth surface finish, availability in several colors, and high flow for easy moldability. The company uses Ixef PARA for the handle and instrument bodies of its new TruTORQ and TruPWR instruments used for critical orthopaedic and spine procedures.
“Our ongoing collaboration with ECA Medical is helping to improve the design and development of single-procedure instruments that can reduce healthcare costs while delivering high precision and performance to support positive outcomes,” says Rose Catherin, sales director, Americas - channel partners & digital sales at Solvay Materials. “The strength and ergonomic properties of our advanced Ixef compounds continue to appeal to industry leaders like ECA Medical that are looking for exceptional materials backed by Solvay’s extensive training, service and support.”
TruTORQ and TruPWR instruments are designed to assist surgeons with the precise fixation of orthopedic and spine implants. They provide audible feedback and tactile feel features to indicate torque achievement. They deliver performance similar to that of reusable instruments while minimizing carbon footprint and lowering costs by eliminating resource-intensive, expensive reprocessing steps that involve harsh cleaning agents, large amounts of clean water for rinsing and repeated steam sterilization.
“There is growing demand worldwide for one-way instruments that are sterile pack and surgery ready to provide clinically robust solutions at a lower lifecycle cost than reusables,” says Lane Hale, president and CEO, ECA Medical Instruments. “To meet this need, we depend on Solvay’s Ixef PARA, which enables us to mold very strong parts that mimic steel yet are less expensive and achieve precise tolerances that are ideal for surgical procedures without creating high costs for inspection. Ixef PARA has helped ECA Medical Instruments achieve the largest market share in the world for single-use instruments, including torque limiters for medical devices.”
Ixef PARA compounds offer metal-like strength, rigidity, and dimensional stability, while providing a high-quality surface. They are optimized for sterilization using high-energy gamma radiation without significant changes in appearance or performance and are available in a range of gamma-stabilized colors. Solvay’s compounds have been evaluated for ISO 10993 limited duration biocompatibility and are supported by a Food & Drug Administration Master Access File.
Latest from Today's Medical Developments
- Children’s National, FDA collaborate to advance pediatric device regulatory tools
- LK Metrology’s eco-friendliness CMMs
- Two patents for microfluidic valves
- AMADA WELD TECH’s blue diode laser technology
- Post-IMTS decline in manufacturing technology orders blunted
- ARS Automation’s FlexiBowl 200
- LMA Consulting urges businesses to restructure supply chains now
- Walter’s WEP01C indexable inserts





