Credit: M4 Division/The Ohio State University
Many new medical devices start as ideas from healthcare providers about how healthcare can be improved. Often, healthcare providers collaborate with medical device manufactures to bring the idea to market and the medical device industry relies on physician input for innovation1.
Now, healthcare providers and medical device manufacturers are closer than ever to directly provide the best solutions to patients through point-of-care (POC) manufacturing. Why now? There are two main reasons: the development of accessible, user-friendly computer aided design and manufacturing (CAD/CAM) methods, such as additive manufacturing; and the clinical benefits of personalized medical devices.
Doctors and engineers at The Ohio State University (OSU) had a vision for the impact of POC manufacturing on healthcare, both by developing new technologies and providing personalized solutions for patients, leading to the formation of Ohio State’s Medical Modeling Materials and Manufacturing (M4) Division. Lead by Kyle VanKoevering, M.D. and Megan Malara, Ph.D., the M4 Division was founded in 2020 as a collaboration between the Center of Design and Manufacturing Excellence (Ohio State College of Engineering) and the Department of Otolaryngology (Ohio State College of Medicine).

With increased demand for personalized medical devices and the accessibility of CAD/CAM technologies, the concept of POC manufacturing (also referred to as in-house manufacturing or hospital 3D printing labs) has emerged in healthcare facilities where in-house labs produce devices intended only for use with the hospital’s patients. This trend is happening across many types of healthcare facilities and geographical locations, with examples including Rady Children’s Hospital-San Diego, the US Veteran’s Health Administration, and Mayo Clinic in the United States, as well as Switzerland’s Universitätsspital Basel and Singapore General Hospital.
Clinical value and example cases from OSU
Personalized devices are used in a variety of ways to benefit patients and the delivery of healthcare solutions. Patients can see benefits from better clinical outcomes2–4, especially for complex cases, and better understanding of disease/choices for treatment5. Furthermore, surgeons have more confidence in the planned surgery, better localize tumors for cancer resection6, and reduce operating time which can save the hospital money7.
POC manufacturing specifically can reduce device production time by simplifying transfer of patient imaging data and eliminating shipping of the final product. It can also improve the physician input required for medical devices personalized to match a patient’s anatomy. In some cases, healthcare facilities can create investigational devices that meet clinical needs where there is no commercially available solution. Additionally, the healthcare setting provides a unique environment for new ideas and feedback on prototyping as well as the ability for clinical testing of developed prototypes.
At OSU, different scenarios are used to meet the clinical need and realize the benefits of POC manufacturing. Examples include:
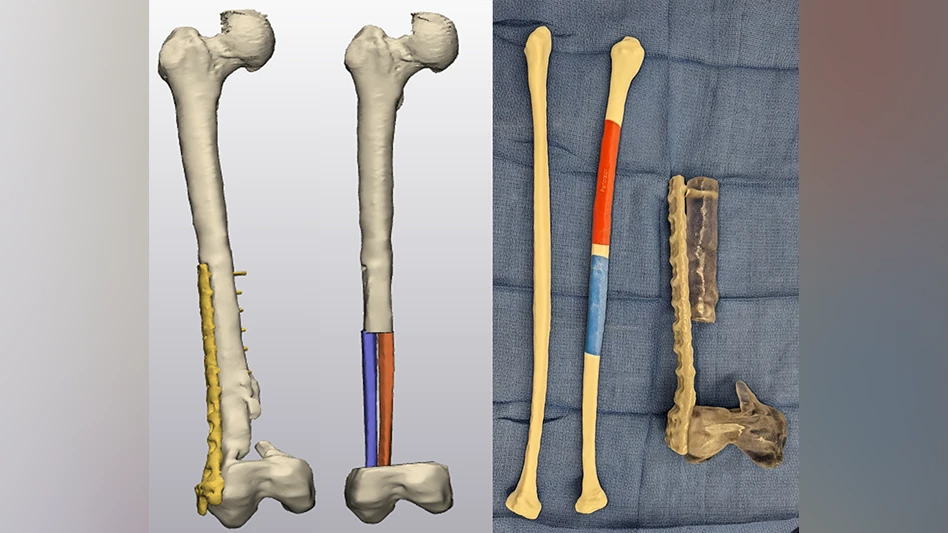
- In-person planning for complex, personalized implants (device manufacturer engineers onsite at healthcare facility), Figure 1
- Improves physician input for personalized devices and feedback to medical device manufacturers and engineers.
- Trained healthcare facility staff utilizing FDA cleared products indicated for POC manufacturing to produce devices8, Figure 2
- Improves physician input and reduces time for patient data transfer and device shipping.
- Clinical trial of investigational device to meet clinical need9, Figure 3
- Enables development of a novel device to improve patient care.
POC manufacturing operations
Regulatory
As POC manufacturing methods are producing medical devices, which are regulated products, there are regulatory considerations that need to be addressed. However, healthcare settings that are practicing medicine, such as hospitals, are not typical environments for medical device manufacturing regulatory bodies to oversee. Physicians usually have some control to decide what is best for their patient under the practice of medicine, such as using medical products off label. In 2021 the US Food and Drug Administration (FDA) released a discussion paper10 on 3D printing in hospitals – indicating different scenarios for POC manufacturing and the responsibilities of the entity involved in each scenario (healthcare facility vs traditional device manufacturer). As the FDA regulatory perspective on POC manufacturing develops, eventually the 2021 discussion paper will be followed by guidance from the FDA that all institutions involved with POC manufacturing will be expected to follow. Additionally, for some institutions, there is the possibility of creating investigational devices that will be used in a clinical trial and overseen by the Institutional Review Board (IRB) of the institution (in cases of non-significant risk devices) or both the FDA and IRB (in cases of significant risk devices).
It is likely that healthcare facilities will employ multiple scenarios and take on varying levels of responsibility depending on the clinical need. At OSU, examples range from manufacturer with full responsibility (example 1, Figure 1, above), manufacturer and healthcare facility sharing responsibility (example 2, Figure 2, above), and healthcare facility assuming full responsibility (example 3, Figure 3, below).
Organization and resources
The organization of POC manufacturing operations varies from institution to institution – sometimes it is housed within medical departments such as radiology or anesthesiology, within medical centers but outside of specific medical departments or, for university hospitals, it may be housed within another entity within the university such as in the engineering department. In other cases, hospitals partner with medical device manufacturers to co-locate their operation within the hospital system.

POC manufacturing requires qualified personnel, software for design and development of devices, and manufacturing equipment (most commonly 3D printers) to create the devices. While it is unclear the specific requirements for healthcare facilities due to developing regulatory guidance, a quality management system is required to ensure that POC devices are safe and effective for their intended use11.
Funding
Many POC manufacturing operations initially gain institutional or grant funding to begin projects and operations. In some cases, philanthropy plays a large role by providing funding for capital equipment or to sustain POC programs. Some programs also receive industry support for projects on aligned interests. Additionally, hospitals can get insurance reimbursement for anatomic models and surgical guides. Since 2019, there are four temporary Category III Current Procedural Terminology (CPT) codes for insurance reimbursement including 055T, 0560T, 0561T, 0562T and ongoing work for them to become Category I codes. Progress from CPT Category III to Category I codes indicates development from emerging to widely adopted technology.
When directly compared to traditional medical device manufacturers which provide solutions for multiple hospitals nationwide, in-house POC manufacturing may struggle with lower volumes and less efficient processes. However, other advantages of POC manufacturing that improve patient care such as quicker delivery times and higher physician oversight or financial models that include philanthropic support and decrease other business costs, may help counterbalance or reduce costs and contribute to wider adoption of patient-specific technologies to improve patient care.
Challenges
As POC manufacturing grows, questions have arisen around evidence for added benefit, applicable regulation, and sustaining these programs financially and operationally. Many challenges are ubiquitous across POC manufacturing labs, though differences exist based on institution and regional locations. While navigating these challenges is not straight forward, the value of close collaboration between medical device manufacturers and healthcare providers has been proven over time. The next years will be exciting to see which clinical applications continue to gain traction and how POC manufacturing develops.
References
- Bergman A, Grennan M, Swanson A. Medical device firm payments to physicians exceed what drug companies pay physicians, target surgical specialists. Health Aff. 2021;40(4):603-612. doi:10.1377/hlthaff.2020.01785
- May MM, Howe BM, O’Byrne TJ, et al. Short and long-term outcomes of three-dimensional printed surgical guides and virtual surgical planning versus conventional methods for fibula free flap reconstruction of the mandible: Decreased nonunion and complication rates. Head Neck. 2021;43(8):2342-2352. doi:10.1002/HED.26688
- Nyirjesy SC, Heller M, von Windheim N, et al. The role of computer aided design/computer assisted manufacturing (CAD/CAM) and 3- dimensional printing in head and neck oncologic surgery: A review and future directions. Oral Oncol. 2022;132:105976. doi:10.1016/J.ORALONCOLOGY.2022.105976
- Sun Z. Patient-Specific 3D-Printed Models in Pediatric Congenital Heart Disease. Children. 2023;10(2). doi:10.3390/CHILDREN10020319
- Santiago L, Volk RJ, Checka CM, et al. Acceptability of 3D-printed breast models and their impact on the decisional conflict of breast cancer patients: A feasibility study. J Surg Oncol. 2021;123(5):1206-1214. doi:10.1002/JSO.26420
- Wake N, Wysock JS, Bjurlin MA, Chandarana H, Huang WC. “Pin the Tumor on the Kidney:” An Evaluation of How Surgeons Translate. Urology. 2019;131:255-261. doi:10.1016/j.urology.2019.06.016
- Ballard DH, Mills P, Duszak R, Weisman JA, Rybicki FJ, Woodard PK. Medical 3D Printing Cost-Savings in Orthopedic and Maxillofacial Surgery: Cost Analysis of Operating Room Time Saved with 3D Printed Anatomic Models and Surgical Guides. Acad Radiol. 2020;27(8):1103-1113. doi:10.1016/j.acra.2019.08.011
- J Mater Res. 2021. doi:10.1557/s43578-021-00270-x
- Patient-specific tracheal stoma plug improves quality of life for tracheostomy patients. BMJ Innov. December 2022:bmjinnov-2022-001028. doi:10.1136/BMJINNOV-2022-001028
- United States Food and Drug Administration. Discussion Paper: 3D Printing Medical Devices at the Point of Care. 2021.
Bastawrous S, Wu L, Strzelecki B, et al. Establishing Quality and Safety in Hospital-based 3D Printing Programs: Patient-first Approach. RadioGraphics. 2021;41(4):1208-1229. doi:10.1148/RG.2021200175
Latest from Today's Medical Developments
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out
- Analysis, trends, and forecasts for the future of additive manufacturing
- BlueForge Alliance Webinar Series Part III: Integrate Nationally, Catalyze Locally
- Robot orders accelerate in Q3
- Pro Shrink TubeChiller makes shrink-fit tool holding safer, easier
- Revolutionizing biocompatibility: The role of amnion in next-generation medical devices
- #56 Lunch + Learn Podcast with Techman Robot + AMET Inc.





