Zsquare
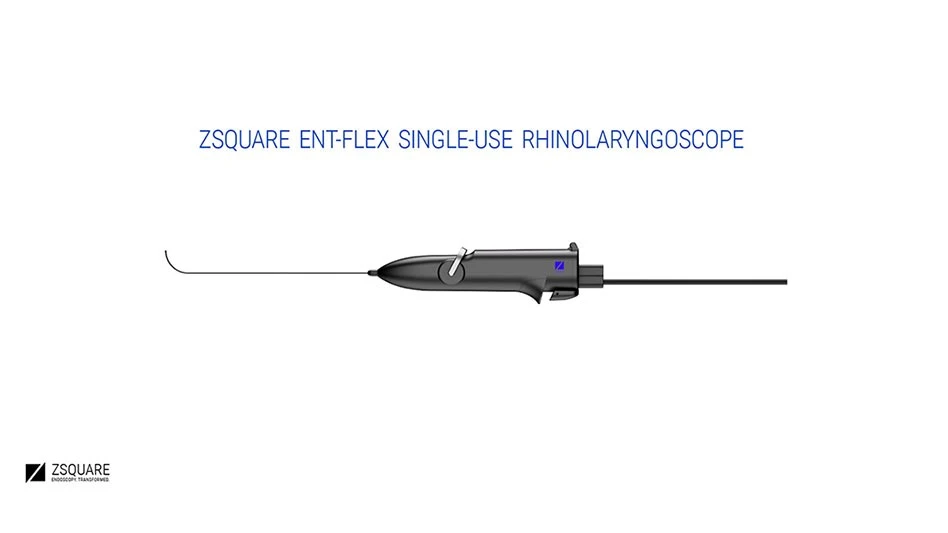
Zsquare, a developer of single-use endoscopes, announced it received U.S. Food and Drug Administration 510K clearance to market its first product, the Zsquare ENT-Flex Rhinolaryngoscope. Its pilot launch in U.S. hospitals and physician practice offices is scheduled for Q4 2022.
The product is indicated for use in diagnostic ENT procedures by way of the nose and throat and is a scalable platform capable of transmitting high-resolution images in flexible, single-use endoscopes. The single-use endoscope uses a hybrid design in which its all-optical disposable shell fully covers a reusable imaging core. The enhanced design provides health professionals with high resolution imaging in single-use endoscopy through its small diameter endoscope shaft. This engineering has several benefits, including improved patient comfort and enhanced diagnostic quality.
The company's patented polymeric imaging fiber’s flexible ultra-thin properties enable smaller endoscopes that can access formerly unreachable anatomical sites without compromising image quality. The hybrid endoscope design enables cross-industry scalability by allowing the same imaging core to be used with different indication-specific disposable shells made for ENT, urology, bronchoscopy, GI, and gynecology.
Latest from Today's Medical Developments
- Birk Manufacturing achieves ISO 13485 recertification
- SW North America's BA 322i twin-spindle CNC
- The role of robotics in precision medical device manufacturing
- Swiss Steel Group’s UGIMA-X machinable stainless-steel sets
- #51 - Manufacturing Matters - The Impact of M&A in MedTech 2024
- Visual Components: 25 years of simulation and programming software innovation
- Zimmer Biomet announces definitive agreement to Acquire Paragon 28
- Discover an innovative technology for EMI/ESD/TVS suppression





