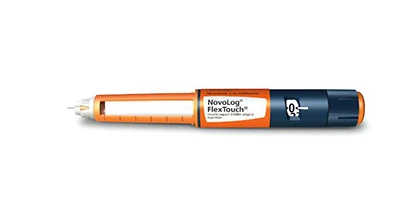
Novo Nordisk officials announce that the U.S. Food and Drug Administration (FDA) has approved the prefilled insulin pens NovoLog (insulin aspart [rDNA origin] injection) FlexTouch and Levemir (insulin detemir [rDNA origin] injection) FlexTouch. FlexTouch is a new prefilled insulin delivery system from Novo Nordisk.
When a dose is dialed with traditional prefilled pens, the push button extends, and at larger doses this can present challenges for the patient. The unique dosing mechanism of FlexTouch ensures the push-button does not extend at any dose and allows insulin to be administered by pressing the low dose force button.
"Novo Nordisk is committed to continuously introducing new solutions that can improve the lives of people with diabetes," says Mads Krogsgaard Thomsen, executive vice president and chief scientific officer of Novo Nordisk. "This approval marks another advancement in insulin delivery and an important milestone for Novo Nordisk."
Novo Nordisk plans to make NovoLog FlexTouch and Levemir FlexTouch available in the United States within the next year. FlexTouch was approved by the European Commission in July 2011 and has launched in several countries, including the United Kingdom, Canada, Denmark, and Japan.
Source: Novo Nordisk
Latest from Today's Medical Developments
- IMTS 2026 runs Sept. 14-19 at McCormick Place in Chicago, Illinois
- Master Bond’s MasterSil 800Med
- ZEISS celebrates 100 years of advancing innovation in the US
- Teleflex sells acute care and urology businesses for $2.03 billion
- HANNOVER MESSE: Where research and manufacturing meet
- What’s next for the design and manufacturing industry in 2026?
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out





