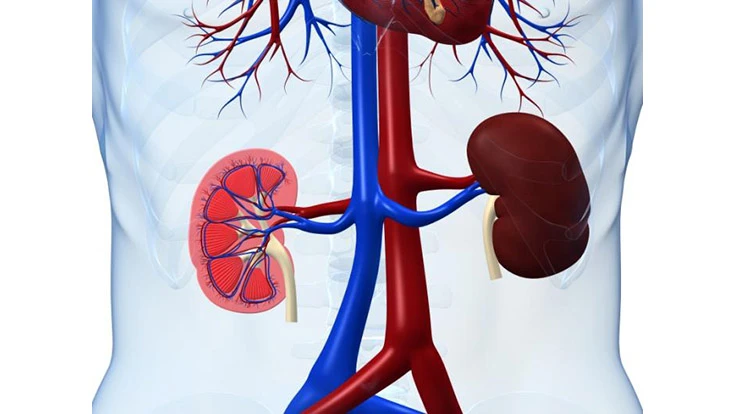
Cleveland, Ohio – According Stratistics MRC, the global nephrology and urology devices is valued at $17.28 billion in 2015 and is expected grow at a CAGR of 7.2% reach $28.27 billion by 2022. Rising occurrence of chronic kidney diseases, increased spending on healthcare among the emerging regions and growing aged population across the world are some of the factors favoring the market growth. Moreover, the growing awareness about the benefits of these devices such as abridged recovery and treatment time is also propelling the global market. On the other hand, the high cost linked with these devices hampers the market.
Dialysis devices commanded the largest market share and BPH treatment devices are anticipated witness the fastest growth during the forecast period. Fresenius was leading the market in dialysis devices followed Baxter Corp. Benign Prostatic Hyperplasia is a major concern and is expected affect around 75% of male over the age of 55, thus driving the market grow. Regionally, Europe is presently leading the market owing the increasing need keep up with the infrastructural requirements in hospital in region. Asia-Pacific is expected grow at a faster pace due the promising medical tourism industry in the region. New Product launch, merger and acquisition, and research and development are the successful strategies followed by the players in the market gain the traction.
Some of the key players in global Nephrology and Urology Devices market are: NikkisCo. Ltd., Boston Scientific, American Medical Systems LLC, Cook Medical, Olympus Medical Systems, Asahi Kasei Corp., Rockwell Medical Technologies Inc., Dornier MedTech, Coloplast, TerumCorp., Fresenius Medical Care, B Braun Group, NiprCorp., Baxter Int’l Inc., NxStage Medical Inc., and C. R. Bard Inc.
Products covered:
- Benign prostatic hyperplasia devices
- Urinary stone treatment
- Prostate cancer
- Urinary incontinence devices
- UI-bulking
- UI-slings
- Urological endoscopes
- Urological catheters
- Dialysis devices
- Erectile dysfunction device
- Nephrostomy
- Pelvic floor repair
- Ureteral access devices
- Endoscopy devices
Type of diseases covered:
- Urinary incontinence (UI) and pelvic organ prolapsed (POP)
- Incontinence products
- Interventional therapies
- Urinary stone
- Open surgery
- Percutaneous nephrostolithotomy (PCNL)
- Laser lithotripsy
- Extra-corporeal shock wave lithotripsy (ESWL)
- Benign prostatic hyperplasia (BPH)
- Holmium Laser Enucleation of Prostate (HOLEP)
- Transurethral radifrequency Needle Ablation of the Prostate (TUNA)
- Stenting (Prostatic Stents)
- Surgery for BPH
- Catheterization
- Transurethral microwave thermotherapy of the prostate (TUMT)
- Chronic kidney disease and renal failure
- Hemodialysis
- Kidney transplant
- Peritoneal dialysis
End users covered:
- Hospitals and clinics
- Ambulatory surgical centers (ASC)
- Home
- Other end users
Regions covered:
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- Italy
- U.K.
- Spain
- Rest of Europe
- Asia Pacific
- Japan
- China
- India
- Australia
- New Zealand
- Rest of Asia Pacific
- Rest of the World
- Middle East
- Brazil
- Argentina
- South Africa
- Egypt
Source: www.wiseguyreports.com
Latest from Today's Medical Developments
- Coating could make medical devices safer for patients
- How robots and cobots can work for you
- Renishaw receives Industry Partner Award
- norelem's modular clamping systems for metrology
- IMTS 2024 Booth Tour: INDEX Corporation
- Robot watched surgery videos then performed with skill of human doctor
- Master Bond’s EP21LSCL-2Med two component non-cytotoxic epoxy
- IMTS 2024 Booth Tour: Tornos Technologies