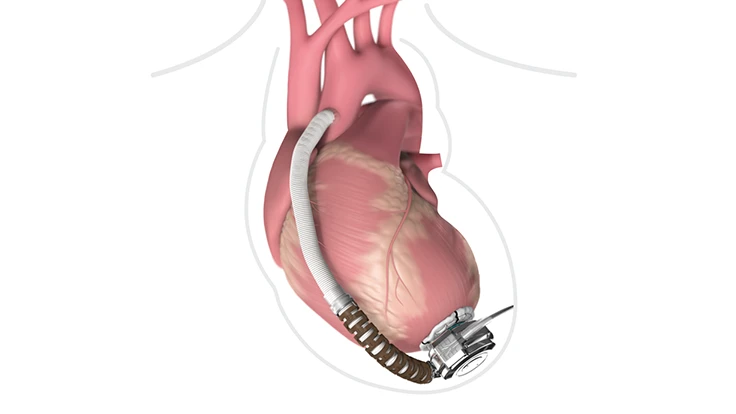
Dublin, Ireland – Medtronic is recalling the HeartWare HVAD because of the possibility for an interruption to occur in the electrical connection between the system’s power source (battery, AC adapter, or DC adapter) and the HVAD controller. This interruption to the electrical connection, which occurs when the power source is still physically connected, is caused by oxidation on the connecting surfaces between the power source connector and the controller’s power source socket. Interruptions to the electrical connection could cause unintended intermittent electrical disconnection, which could result in a pump stop. A pump stop could cause patient harm such as exacerbation of heart failure symptoms, or symptoms such as mild weakness, dizziness, anxiety, nausea, loss of consciousness, or death.

The United States Food and Drug Administration (FDA) has classified Medtronic plc's recent voluntary urgent field action related to the HeartWare HVAD System unexpected power source switching as a Class I recall. Class I recalls describe situations where there is reasonable risk of serious adverse health consequences or death.
There have been no confirmed reports of catastrophic harm associated with this issue. The per patient probability of serious adverse events due to this issue is approximately 0.003. This recall affects 16,399 HeartWare Ventricular Assist Device (HVAD) Systems implanted as of May 22, 2018.
In a notification letter distributed in May 2018, Medtronic alerted clinicians worldwide about a potential transient interruption of the electrical connection between a HeartWare HVAD System power source and the HVAD Controller, which results in unintended switching to the device's secondary power source and could cause the System to momentarily stop and restart. In addition, unintended power switching can result in unexpected audible tones ("beeping"). This beeping, which occurs when the electrical connection interruption automatically resolves, may confuse the patient or caregiver, as the Controller may display sufficient battery capacity or AC/DC connectivity at the time of the audible tone. Also, a Critical Battery Alarm may be momentarily incorrectly displayed due to this phenomenon.

A full list of affected devices is available in the FDA recalls database.
[06/01/2018 - Recall Notice - FDA]
Recommendation
Medtronic sent a letter advising hospitals and physicians to:
- Reinforce the importance of always ensuring two power sources are connected at all times
- Reinforce best practice guidance for managing power sources when going to sleep and awakening
- Instruct patients to report any persistent, unexpected audible tones to the VAD team for additional instructions. In addition, FDA reminds patients to call 911 if they are experiencing a medical emergency.
Background
The HeartWare Ventricular Assist System (HVAD) is indicated for use as a bridge to cardiac transplantation in patients who are at risk of death from end-stage left ventricular heart failure. It functions as a pump that helps the heart deliver blood to the rest of the body. The HeartWare System is designed for in-hospital and out-of-hospital settings, including transportation via fixed wing aircraft or helicopter. These indications have been expanded to include HVAD use for myocardial recovery, or as destination therapy in patients for whom subsequent transplantation is not planned.
Latest from Today's Medical Developments
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out
- Analysis, trends, and forecasts for the future of additive manufacturing
- BlueForge Alliance Webinar Series Part III: Integrate Nationally, Catalyze Locally
- Robot orders accelerate in Q3
- Pro Shrink TubeChiller makes shrink-fit tool holding safer, easier
- Revolutionizing biocompatibility: The role of amnion in next-generation medical devices
- #56 Lunch + Learn Podcast with Techman Robot + AMET Inc.





