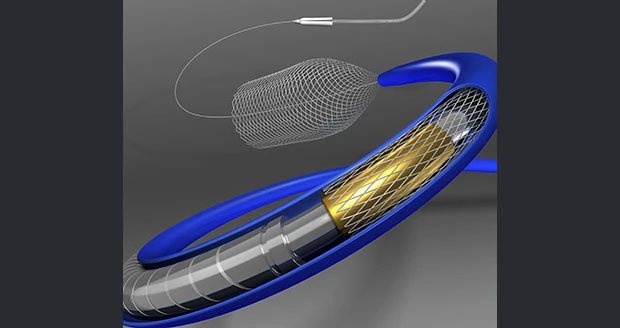
Dublin, Ireland – Medtronic plc officials announced that the company has received U.S. Food and Drug Administration (FDA) approval for its Pipeline Flex embolization device. Available through a limited U.S. launch in the coming weeks, Medtronic's latest-generation flow diversion device represents an unrivaled advancement in large and giant brain aneurysm treatment.
“Flow diversion has been a major breakthrough therapy for large or giant wide-necked brain aneurysms that are complex and have considerably higher risk of rupture and higher rates of complication with conventional treatment," said Dr. Ricardo Hanel, neurosurgeon, director of stroke and Cerebrovascular Center at Baptist Health in Jacksonville, Florida. “With thousands of patients successfully treated with Pipeline Embolization Device, the Pipeline Flex’s innovative delivery system will result in further advancing endovascular treatment and care.”
Designed to divert blood flow away from an aneurysm, the Pipeline Flex embolization device features a braided cylindrical mesh tube that is implanted across the base or neck of the aneurysm. The device cuts off blood flow to the aneurysm, reconstructing the diseased section of the parent vessel.
“The Pipeline Flex embolization device is the next advancement in flow diversion, combining our clinically-proven braid design with a new delivery system designed to offer improved accuracy and control when performing these advanced procedures inside the brain,” said Brett Wall, president, Neurovascular, Medtronic. “We are excited to bring new value to our medical community and patients."
In the United States, the Pipeline Flex device is intended for use for the endovascular treatment of complex intracranial aneurysms that are not amenable to treatment with surgical clipping and are attached to parent vessels measuring between 2.5mm and 5.0mm in diameter. An estimated 500,000 people throughout the world die each year caused due to ruptured brain aneurysms, and half the victims are younger than 50 years of age.
The first-generation Pipeline embolization device has been used to treat patients in the United States since it was approved by the FDA in 2011. This product is part of the Neurovascular portfolio in Medtronic’s Restorative Therapies Group.
Source: Medtronic
Latest from Today's Medical Developments
- Manufacturing technology orders fall for 3rd year
- Soft joint model for robots
- TANAKA PRECIOUS METAL TECHNOLOGIES’ Visi Fine
- Auxilium Biotechnologies prints medical devices on the International Space Station
- KYOCERA SGS Precision Tools’ APEX Application Expert
- North American robotics market holds steady in 2024 amid sectoral variability
- Evident’s DSX2000 digital microscope
- Ferrocene becomes first Rust toolchain to achieve IEC 62304 qualification





