AdobeStock_453661303
Advancements in medical technology continue to advance the quality of life for people globally. As I’ve written before about Parkinson’s disease and the impact it had on my father, while these developments came to late to help him while he was alive, the advantages this brings to those suffering from Parkinson’s is just amazing.
As you read below, I’m also highlighting the most recent approval OrthoNovis got for its wrist fracture system. Commonly occurring fractures requiring surgery and plating are now able to be treated in a more economical manner with improved patient outcomes. Through the advanced research and development the medtech community does – along with the advanced manufacturing technology to produce these medtech devices – everyone benefits.
Medtronic plc received U.S. Food and Drug Administration (FDA) approval of BrainSense Adaptive deep brain stimulation (aDBS) and BrainSense Electrode Identifier (EI).
There is no cure for debilitating neurological conditions like Parkinson's, however, deep brain stimulation (DBS) has been transforming the lives of people with Parkinson's and other neurological disorders for more than 30 years. DBS is similar to a cardiac pacemaker, but for the brain. It uses a surgically implanted neurostimulator via a minimally invasive procedure to transmit electrical signals to specific parts of the brain affected by debilitating neurological disorders.

Medtronic has enhanced its Percept DBS neurostimulators with BrainSense Adaptive technology, introducing aDBS for people living with Parkinson's. This feature personalizes therapy based on a patient's brain activity in real time – in clinical settings and in daily life. It provides enhanced therapy personalization for symptom control that automatically adjusts, minimizing the need for patients to manually adjust stimulation.
The U.S. FDA approval also includes the Medtronic BrainSense Electrode Identifier (EI), which helps reduce patient time spent in clinic to program their DBS settings. By using EI, clinicians can conduct an accurate and precise initial programming, 85% faster compared to traditional electrode selection. To learn more about Medtronic DBS with BrainSense technology, visit website.
OrthoNovis Inc. received U.S. Food and Drug Administration (FDA) clearance to market its BPS Wrist Fracture System. The 510(k) clearance authorizes OrthoNovis to market a line of locking wrist plates designed for the fixation of certain fractures, fusions, or osteotomies in the distal radius.
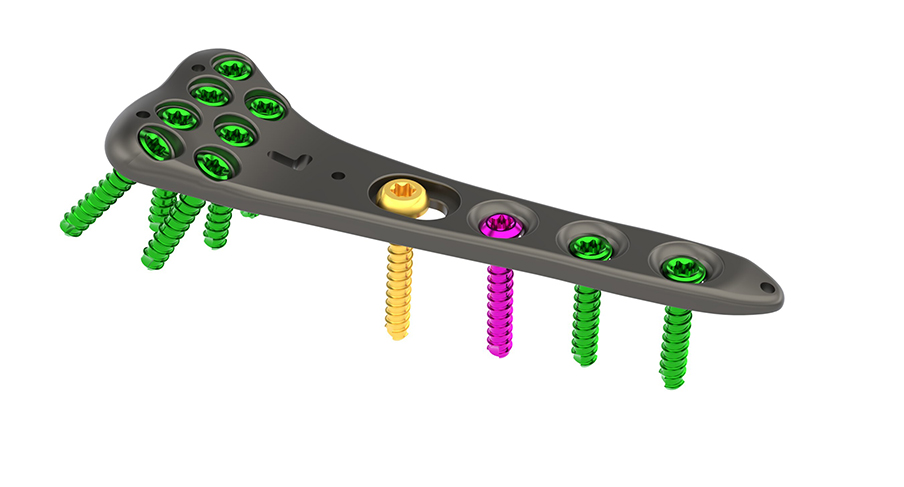
"This milestone will allow OrthoNovis to compete in the rapidly growing US orthopedic fracture fixation market," says Ken West, president, and CEO. "Our BPS Wrist Fracture System is a critical step in the build-out of our complete line of orthopedic implants featuring our patented technologies and financially responsible pricing. We are well positioned to grow and will be releasing several new product lines that will address the treatment and fixation of commonly performed Orthopedic procedures."
Latest from Today's Medical Developments
- A 30-year journey through CAM: Insights on simulation technology
- Behringer Saws’ HBE663A Dynamic horizontal bandsaw
- North America's supply chains face sharp decline due to tariffs
- Experience precision: GF Machining Solutions' CUT F Series wire EDM
- Mastering high-temp alloys with Kennametal Inc.
- Integer expands operations in Salem, creating 83 jobs
- Siemens unveils new Teamcenter X: Revolutionizing SaaS PLM for all manufacturers
- 3 Questions with an Expert with Allied Machine & Engineering