Livonia, Michigan – Officials from nanoMAG LLC, an advanced materials manufacturing company and the developer of a novel bioabsorbable magnesium alloy technology, announce the high strength bioabsorbable Magnesium alloy, BioMg 250, is now available in wire form for biomedical orthopaedic procedures.
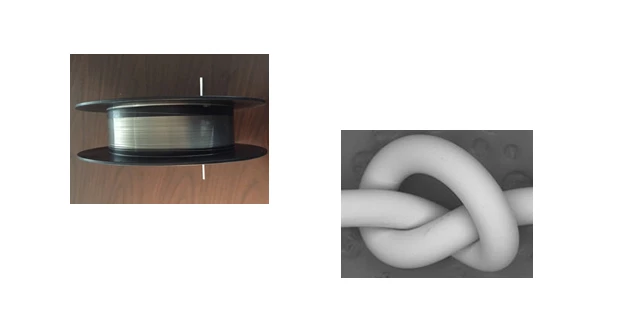
Building on the wire-making skills of Fort Wayne Metals, wire is now available from nanoMAG at 1.1mm and 0.3mm diameter. Yield strength can be varied between 300MPa and 370MPa. The ductility of the BioMg 250 wire is demonstrated by the tight knot tied in the 0.3mm wire. Potential applications in orthopaedics using wire include K wire, suture wire and cables, tension band wiring, mesh for stents and fiber-mesh for reinforcing hydroxyapatite bone-filling cements.
The nanoMAG strategy is to partner with industry leading biomedical implant makers to design and develop metallic implants to be bioabsorbable, according to Steve LeBeau, president, nanoMAG LLC. These implants are developed using proprietary alloys of magnesium and other compatible elements which provide the appropriate strength and bone support while healing occurs, after which the implant dissolves and is absorbed by the body. The alloy content of BioMg 250 is designed to be free of Aluminum and Rare Earth elements; rather it is microalloyed with elements that are nutrients in the body and which foster new bone growth. At the same time, these microalloying elements are balanced to provide high strength for bone fixing along with optimum bioabsorption rate to obviate any need for secondary removal operations. nanoMAG partners have suggested applications such as screws, staples, tacks, wire, rods, plates, and 3D shapes for ligament fixations, craniofacial implants, and small bone implants. “These new magnesium parts could radically change how orthopaedic implants are made and used,” said LeBeau.
nanoMAG is seeking potential partners interested in collaborating on new product development projects. The materials are being tested as part of an ongoing internal program to obtain FDA regulatory approval for the use of bioabsorbable magnesium alloy implants in orthopaedic applications.
This technology development was supported in part by the National Science Foundation under Contract No. 0847198 (Project Manager, Dr. Prakash Balan).
For more information, contact Dr. Steve Lebeau at slebeau@nanomag.us; Tel: 734.261.2800.
Source: nanoMAG LLC
Latest from Today's Medical Developments
- Medtronic: 5 healthcare tech trends for 2025
- Norman Noble launches enhanced laser welding capabilities; expands micromachining in Florida
- What you need to know about CMMC requirements
- CO2 footprint of a machine tool
- Ainos unveils AI Nose for robotics
- Keep up with the latest in design and manufacturing through free webinar
- MedCon 2025 takes place April 23-25 in Columbus, Ohio
- Portescap’s miniature motor capabilities at MD&M West 2025