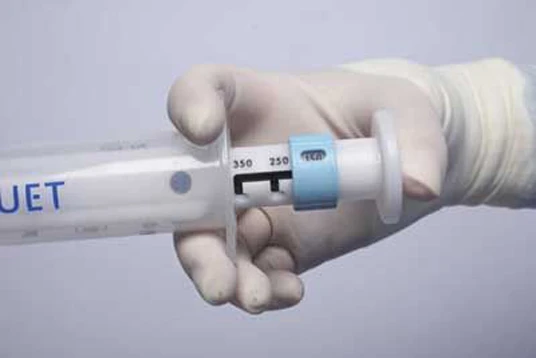
MAQUET Cardiovascular LLC has launched, in the United States, the new VASOSHIELD Pressure Controlling Syringe for use during Coronary Artery Bypass Graft (CABG) surgery. The VASOSHIELD syringe limits internal "flushing" pressure and controls vessel distension when harvested grafts are irrigated in preparation for use in bypass surgery. Avoiding excessive pressure and limiting distension help maintain internal vessel integrity, which has been shown in clinical studies to be an important factor affecting conduit quality and long-term graft success. The new VASOSHIELD syringe will be demonstrated to cardiothoracic surgeons at the American Association of Thoracic Surgeons (AATS) 90th Annual Meeting, which is taking place from May 1-5, 2010 in Toronto, Canada.
"Accurately assessing and controlling pressure in grafts with a standard syringe can be difficult," says Michael J. Mack, M.D., Research Director, THE HEART HOSPITAL Baylor Plano. "We look forward to using the VASOSHIELD syringe because we expect it to assist us in the meticulous handling and preservation of vein grafts, which is vitally important to graft patency and positive patient outcomes. In addition, we look forward to gathering the data to help show the clinical benefit this new tool will provide. We believe it will complement the data we will obtain from the OPTION trial."
Dr. Mack is an investigator for the OPTION (Optimal Improvement of Vein Graft Patency Long Term by the Implementation Of Novel Endoscopic Harvesting Techniques) study. This clinical trial is designed to evaluate the equivalence of Endoscopic Vessel Harvesting (EVH) in CABG surgery compared with historical data for open vein harvesting.
When a vessel is prepared for use as a bypass graft it is frequently distended with a standard syringe to flush and check for leaks. If flushing pressure inside the vessel is not controlled, it has been observed to reach as high as 600 mm Hg, inadvertently damaging the internal vessel surface. By limiting the maximum pressure that can be applied during graft distension, this type of injury may be prevented, thereby improving the overall quality and patency of the graft long term.
The VASOSHIELD Syringe allows the user to select one of three settings -- 150mm, 250mm or 350mm Hg - so that flushing pressure is adjusted in pre-set increments and uninterrupted flow is delivered during vein preparation. It has been clinically shown that vessels flushed at controlled, lower pressures more effectively overcome spasm, maintain their endothelial and medial layers, and are more likely to provide a high-quality conduit for bypass surgery.
The VASOSHIELD Pressure Controlling Syringe is part of the VASOVIEW family of products from MAQUET Cardiovascular, which include a range of EVH technology options to perform minimally invasive vessel harvesting procedures.
"As a leader in the field, MAQUET continues to advance cardiac bypass surgery techniques by investing in technologies that help improve patient outcomes and enhance quality of life," says Patrick Walsh, President of the Cardiac Surgery Business Unit at MAQUET Cardiovascular. "The new VASOSHIELD syringe is yet another example of our commitment to supporting physicians with tools that optimize graft quality and improve standards of care."
About Vessel Harvesting
Vessels for bypass surgery may be retrieved using open surgery or through minimally-invasive endoscopic vessel harvesting (EVH). During EVH, a healthy blood vessel, such as the saphenous vein from the leg or the radial artery from the arm, is removed through a small incision for use during CABG surgery to bypass a blockage in the heart. Open vein harvesting requires a lengthy incision that is often extremely painful and prone to healing complications. EVH is considered a standard of care in the United States, with 80 percent of all CABG surgeries utilizing this technique.
About MAQUET Cardiovascular
MAQUET Cardiovascular was formed in 2003 and is a leader in providing innovative products for cardiac surgery, vascular intervention and cardiac support to hospitals and clinics and the cardiac surgeons, interventional cardiologists, perfusionists and other healthcare professionals who care for patients with cardiovascular disease. MAQUET Cardiovascular is focused on providing clinicians with future-oriented technology that fits into their daily practice and improves the therapeutic management of patients. MAQUET Cardiovascular continues to invest in the development of innovative technologies and solutions that advance clinical practice, improve patient outcomes and enhance quality of life.
MAQUET Cardiovascular provides healthcare professionals with products in four business units: Cardiopulmonary (perfusion products), Cardiac Surgery (clampless beating heart and endoscopic vessel harvesting), Vascular Interventions (grafts for vascular surgery), and Cardiac Assist (intra-aortic balloon counterpulsation therapy).
Latest from Today's Medical Developments
- Best of 2024: #4 Article – Sticking to the basics
- Best of 2024: #4 News – Point of care manufacturing
- Best of 2024: #5 Article – Accelerating medical device development with freeform injection molding
- Best of 2024: #5 News – Complexity, the enduring enemy of medical cybersecurity
- Best of 2024: #6 Article – Closing the global product information gap
- Best of 2024: #6 News – NUBURU enters medical device market with order Blueacre Technology
- Season's greetings
- Best of 2024: #7 Article – Synchronized machining processes for medtech