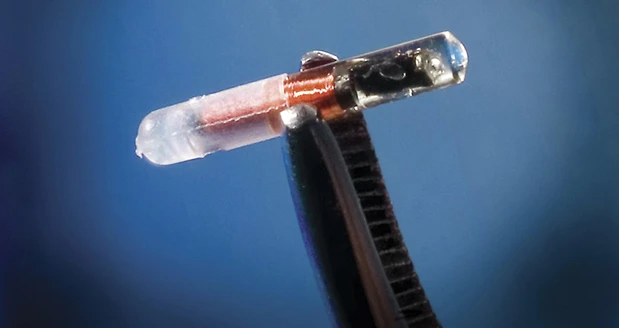
Delray Beach, Florida – Officials from VeriTeQ Corp., a provider of implantable medical device identification and radiation dose measurement technologies, announced that the Food and Drug Administration has released draft guidance that names radio frequency identification (RFID) as a technology solution to comply with the direct part marking requirements of the FDA’s final rule for unique device identification of medical devices. VeriTeQ’s Q Inside Safety Technology is an FDA cleared implantable RFID microtransponder that can be used to identify implantable and reusable/reprocessable medical devices.
In 2013, the FDA released a final rule requiring a unique device identification system designed to adequately identify medical devices through distribution and use. In most cases, the final rule requires a unique device identifier (UDI) to be included on device labels and packages. In addition, the final rule states that a device that must bear a UDI on its label must also bear a permanent marking providing the UDI on the device itself if the device is intended to be used more than once and intended to be reprocessed before each use. Although subject to change, the recent 2015 draft guidance states that acceptable methods to directly mark a device with a UDI include affixing a permanent tag such as an RFID tag to the device.
VeriTeQ’s FDA cleared Q Inside Safety Technology acts as an electronic serial number in breast implants and other implantable and reusable medical devices to provide physicians and patients with access to secure online databases to retrieve device-specific data, such as serial number, manufacturer name, date of manufacture, lot number, volume, size, and other data from the medical device manufacturer. Q Inside Safety Technology may also provide an extra level of protection to the patient in the event of a recall or other safety event.
Scott R. Silverman, chairman and chief executive officer of VeriTeQ, stated, “We are currently working with some of the leading breast implant manufacturers to help them understand that Q Inside Safety Technology not only helps them meet the FDA’s requirement for direct part marking of breast implant sizers and other reusable/reprocessable medical devices, but also offers added value through its ability to help provide long-term outcome data and other healthcare analytics. We also believe that our Q Inside Safety Technology can provide similar benefits to other medical device makers, including manufacturers of artificial joints and vascular ports.”
Source: VeriTeQ
Latest from Today's Medical Developments
- IMTS 2026 runs Sept. 14-19 at McCormick Place in Chicago, Illinois
- Master Bond’s MasterSil 800Med
- ZEISS celebrates 100 years of advancing innovation in the US
- Teleflex sells acute care and urology businesses for $2.03 billion
- HANNOVER MESSE: Where research and manufacturing meet
- What’s next for the design and manufacturing industry in 2026?
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out





