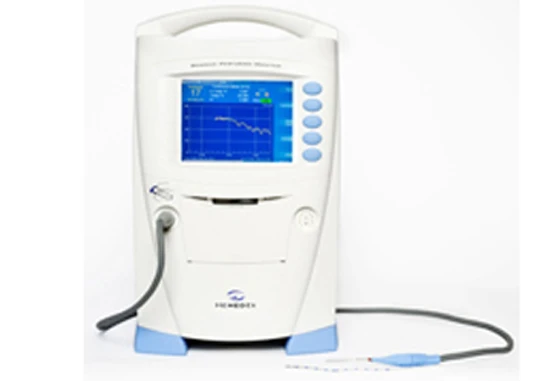
Cogmedix, a US-based, FDA compliant medical device manufacturer, will manufacture the second generation of medical devices for Hemedex Inc.
"Hemedex Inc. designs proprietary, safety critical devices, so it is essential for their company to work with a manufacturer they can trust," says Matt Giza, general manager, Cogmedix. "Working with Cogmedix in the past, Hemedex experienced our flexibility and dedication to FDA compliant, reliable production for safe and cost effective medical device technologies."
Hemedex, the developer of a proprietary platform to quantify tissue perfusion, selected Cogmedix to manufacture the first generation of its Bowman Perfusion Monitor, which calculates, stores and displays perfusion data. After great success in clinical and neurosurgical applications with this monitor, Hemedex will manufacture its second generation of the product with Cogmedix as well.
"Cogmedix is extremely collaborative, professional, and communicative at every step of the manufacturing process." reports Lisa Coffey of Hemedex. "These qualities make the company more than just a vendor-we continue to consider Cogmedix and the Coghlin Companies integral partners in our organization."
About Hemedex, Inc.
Hemedex, Inc., was founded in 2000 to commercialize a proprietary platform technology conceived at the Massachusetts Institute of Technology (MIT) to quantify tissue perfusion (i.e., blood flow at the capillary level). This technology addresses the need for real-time, prognostic data in complex medical and surgical procedures. These complex procedures often involve compromised vasculature and other serious pathophysiology, resulting in high rates of morbidity, delayed recuperation, poor patient outcome and higher overall costs. Hemedex's patented technology can provide an early warning of impending tissue ischemia, and it can help target appropriate therapies rapidly and accurately. In doing so, it aides in stemming some of the ill effects listed above by providing clinicians with a powerful tool to generate valuable prognostic data.
About Cogmedix
Cogmedix is a US-based, FDA compliant medical contract manufacturer. A subsidiary of Coghlin Companies Inc., Cogmedix focuses on the production of Class I medical devices and Class II medical devices for the critical care, home healthcare, emergency room, and industrial laboratories markets. Cogmedix focuses on aligning itself with companies seeking a compliant, conscientious, cost effective, domestic manufacturing resource to assist them with medical contract manufacturing. For more information about Cogmedix, please visit www.cogmedix.net.
About Coghlin Companies, Inc.
Coghlin Companies, Inc. is a privately held company in Worcester, Massachusetts spanning four generations for over 125 years. Subsidiaries include: Columbia Tech, which provides turnkey contract manufacturing services to a diverse customer base, including OEM's in the telecommunication, semiconductor, machine tool, medical device, alternative energy, data storage, and networking industries; DCI Engineering, an engineering services company; Coghlin Precision, a precision machining and certified welding company; and Cogmedix, an FDA compliant medical and clinical device contract manufacturer. For more information, please visit www.coghlincompanies.com.
Latest from Today's Medical Developments
- Humanoid robots to become the next US-China battleground
- Air Turbine Technology’s Air Turbine Spindles 601 Series
- Copper nanoparticles could reduce infection risk of implanted medical device
- Renishaw's TEMPUS technology, RenAM 500 metal AM system
- #52 - Manufacturing Matters - Fall 2024 Aerospace Industry Outlook with Richard Aboulafia
- Tariffs threaten small business growth, increase costs across industries
- Feed your brain on your lunch break at our upcoming Lunch + Learn!
- Robotics action plan for Europe





