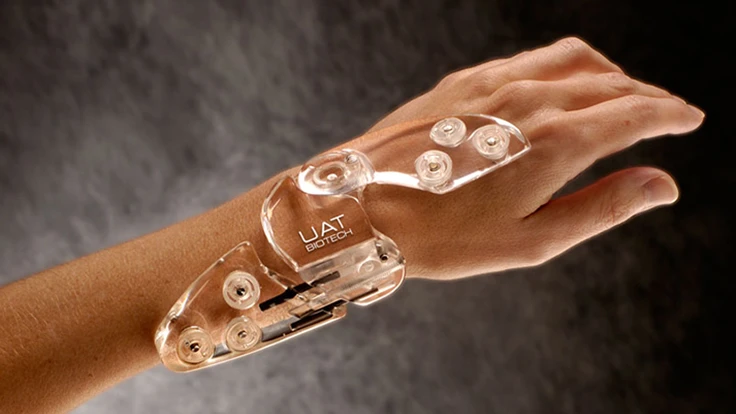
Tampa, Florida – Umbra Applied Technologies Group Inc. announced that its next generation bone fixator, ClearView G2 is ready for release with initial pilot surgeries to be completed over the course of the next 90 days.
Five years ago, through a strategic partnership with GE, Umbra Applied Technologies created the ClearView wrist fixator. The ClearView fixator was the world's first medical grade Lexan based wrist fixator that produced clearer X-ray views of fractured bone reduction and pin placement. The paradigm shifting device further raised the bar by being radiolucent thus reducing the amount of necessary times a clinical staff and patient needed to be exposed to radiation. This device additionally revolutionized the industry by producing a fixator that was lightweight, radiolucent, eliminated the need to modify clothing when worn, fully adjustable post-operative surgery and designed with a unique ball and pin holder assembly enabling pins to be inserted and fixed within a 30-degree arc.
Chairman and Chief Executive Officer Alex Umbra remarked, “Umbra Applied Technologies has successful improved its ClearView wrist fixator to be more versatile so that our unique approach to external bone fixation could be implemented across a wider segment of the orthopedic community. With the development of our ClearView G2 we now have a design that can be used on all small bones such as those found in the feet as well as the hands.”
The ClearView G2 facilitates function while shortening treatment duration by addressing trauma or limb reconstruction in a way that enhances the human body's natural healing process and works within the parameters of a patient's practical daily life. UAT's G2 fixator is the most versatile and advanced product available to be used on applications that include post traumatic and congenital limb reconstruction, limb salvage, complex arthrodesis, management of osteomyelitis and bone defects, and deformity correction. UAT's advanced research arm was able to take their already cutting-edge ClearView fixator and propel it years ahead of its competitors by addressing osteo-healing from the perspective of the human body's biology not mechanics. When pressed to describe how the G2 is better than its competitors, Mr. Umbra responded, "We have been able to enhance a method for osteogenesis that relies on a percutaneous approach with minimal trauma to the limb, closed anatomic fracture reduction, and unheard of bony stability that allows early weight bearing. We have done this with a low profile, low impact device that not only reduces the amount of X-rays a patient may need but we have done it in a way that allows for one device to be used in more than one part of the anatomy. It represents the very best of UAT design and engineering".
The ClearViewG2 is scheduled to go into production in October of this year.
Source: Umbra Applied Technologies Group
Latest from Today's Medical Developments
- VersaBuilt’s CNC automation possibilities
- ROBOTICS AWARD 2025
- There’s still time to register for this week’s manufacturing industry webinar!
- Arterex acquires Phoenix S.r.l., a leading Italian medical device solution provider
- FAULHABER’s expanded portfolio of high-performance DC motors
- NAMSA will acquire WuXi AppTec facilities in Minnesota and Georgia
- Tolomatic’s Drive Integration Tool
- Cutting Edge Innovations: Maximizing Productivity and Best Practices with Superabrasives





