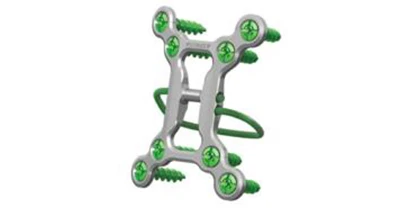
Pioneer Surgical Technology Inc., a leader in innovative medical devices, announces its first clinical use of the Tritium Sternal Cable Plating (SCP) system for closing median sternotomies following an open-heart procedure.
The procedure was performed at St. Rose Dominican Hospital in Henderson, NV, by Dr. Robert Wiencek who notes, “The best part of the [Tritium SCP] system is that it takes the best of both worlds and puts it into one device.”
He continues, “Rather than relying on traditional cerclage cables or anterior plates alone, this new cable plating system provides the benefit of having both systems integrated into one simple technique. You can pull the cables tight, bring the sternum together, and then screw it all down. This eliminates micro-motion in the sternum and combines the best of both closure methods into this single device.”
The Tritium SCP system enhances the stability and strength of traditional sternal closure techniques by utilizing a unique load-sharing concept. The device incorporates the advantages of cerclage cables and cancellous screws, integrated with a low profile plate. “This unique combination creates a load-sharing design that uses cable tension to provide circumferential compression across the median sternotomy,” says Peter Didyk, director of sales and marketing, Pioneer Surgical. “The device complements our current Sternal Cable system by offering additional options to accommodate surgeon preferences and better address variances in patient anatomy.”
Pioneer Surgical received FDA clearance for the Tritium SCP system in October.
"Pioneer celebrated its 20th anniversary last year and this key product launch is a tremendous way to celebrate our past success and focus on our continued growth in and outside the United States,” states Shane Ray, executive vice president of surgical solutions and biologics, Pioneer Surgical Technology. “Within a year, our engineering, marketing, sales, and manufacturing teams took this idea from a napkin drawing to an FDA cleared product with many benefits. We are enthusiastic about our continued service to the Cardio Thoracic community, surgeons, and patients.”
Latest from Today's Medical Developments
- Star Cutter incorporates TRU TECH brand into grinding technology portfolio
- WENZEL’s expanded LH CMM series
- Digital Transformation in 5-Axis Machining
- Viant expands medical device solutions
- MIDACO’s automatic 4-pallet changer with trunnion system
- Breaking Free of the Additive Manufacturing Echo Chamber
- Boston Scientific to Acquire Cortex
- Don’t miss this month’s Manufacturing Lunch + Learn!