Teknic

Amid the COVID-19 pandemic, the world was experiencing critical shortages. As hospitals ran out of ventilators, doctors were turning to anesthesia workstations, BiPAP machines, and CPAP machines to help ventilate patients. As doctors exhausted their supplies, they were scrambling to find alternatives.
At first, the solution may seem obvious – produce more ventilators – but by the time manufacturers are able to scale up production to meet current and future demand, it would be too late.
Given that scaling up conventional ventilators will take too long, medical professionals need additional solutions. At some point, the only remaining option will be to manually ventilate patients with Ambu bags and hope that conventional ventilators become available. Although Ambu bags are readily available, there aren’t enough trained clinicians to operate these devices for every patient in need.
Operating an Ambu bag requires a clinician to actuate the bag about 10 to 30 times per minute without stopping – an action that quickly becomes tiring. When one clinician fatigues, a new one must take over, and this cycle must continue until the patient recovers or a ventilator becomes available.
Teknic worked on a project that automates the operation of Ambu bags. As an original equipment manufacturer (OEM) with extensive experience working on U.S. Food and Drug Administration (FDA) medical applications, our team was aware of many unique requirements for this application.
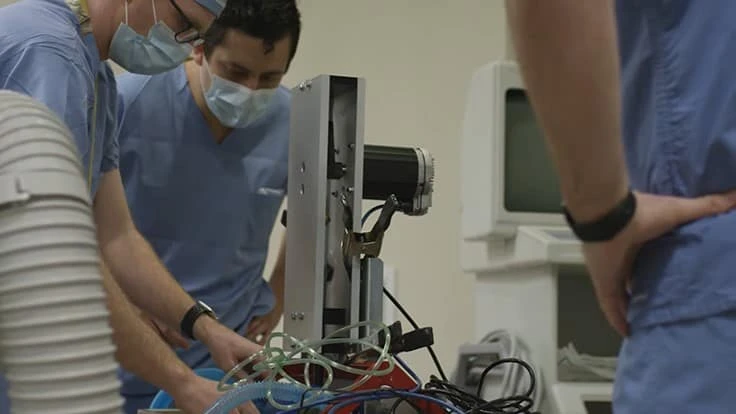
In mid-March, a team including Dr. Stephen Richardson (an anesthesiologist at the University of Minnesota), Jim McGurran (an engineer from MGC Diagnostics), and a small group from the Earl E. Bakken Medical Devices Center conceived the idea of a one-armed robot (more technically, a single-axis linear actuator) to automate human ventilation using an Ambu bag.
When the team had an early working prototype, they contacted Teknic (on a recommendation from Digi-Key, a large electronics distributor) for advice on the project’s motion control requirements. In just more than two weeks, our collective team brought the device all the way through concept, prototype, and production.
The machine, internally nicknamed Ambu-bot by Teknic, was not designed to replace ventilators. Rather, it was designed to automate the manual ventilation typically performed by medical personnel so that clinicians in over-stressed hospitals can treat other patients.
We left out sophisticated adjustments and sensors commonly found (and required) in conventional ventilators to drastically speed up the design, prototyping, testing, and production to meet the urgent need. The machine used one of Teknic’s ClearPath servos, but was designed for a wide range of servo or stepper motors.
While using this machine, clinicians will be required to monitor patients more closely than patients on a conventional ventilator. However, this machine does all the required manual labor consistently and continuously, so a clinician can monitor multiple patients at the same time to ensure that each patient’s vitals stay in an acceptable range.
The FDA formally granted Emergency Use Authorization (EMU) to Boston Scientific and the University of Minnesota, under the official name Coventor Automatic Adult Manual Resuscitator Compressor. Boston Scientific announced plans to initially build 3,000 units, and then more as needed.
Problems solved:
- Be reliable and capable of running 24/7
- Have adjustable speed control
- Have adjustable compression (stroke volume)
- Be easy to build and have an open-source design
- Be ready for scaled up production (1,000 per week) in two weeks, and mass production (10,000 per week) within a few weeks after that
- Be simple in concept and universally applicable
- Be easily used by medical personnel with minimal training time
Mechanical solution overview:
- NEMA 34 Motor/Drive: CPM-MCVC-3441S-RLN
- Standard BVM (“Ambu”) Manual Resuscitator Bag
- Motor-driven compression rod
- Cam-driven mechanics
- 3D-printed (or machined) gears
Development timeline:
- Day 1: Initial Prototype Design
- Day 2: First Prototype Trial
- Day 4: Functional Test at the University of Minnesota
- Day 8: Project Iterations and Pre-Production
- Day 11: FDA Emergency Use Authorization Application and Production
- Day 15: Production Ramps Up and Boston Scientific Boosts Production
- Day 20: Positive Initial Review from the FDA
- Day 28: FDA Emergency Use Authorization Granted
Project partners:
Teknic would like to give special thanks to all of the team members who participated on this project:
- The University of Minnesota Medical School
- The Earl E. Bakken Medical Devices Center
- MGC Diagnostics
- Digi-Key
- Proto Labs
- Appareo Systems
- Boston Scientific
Latest from Today's Medical Developments
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out
- Analysis, trends, and forecasts for the future of additive manufacturing
- BlueForge Alliance Webinar Series Part III: Integrate Nationally, Catalyze Locally
- Robot orders accelerate in Q3
- Pro Shrink TubeChiller makes shrink-fit tool holding safer, easier
- Revolutionizing biocompatibility: The role of amnion in next-generation medical devices
- #56 Lunch + Learn Podcast with Techman Robot + AMET Inc.





