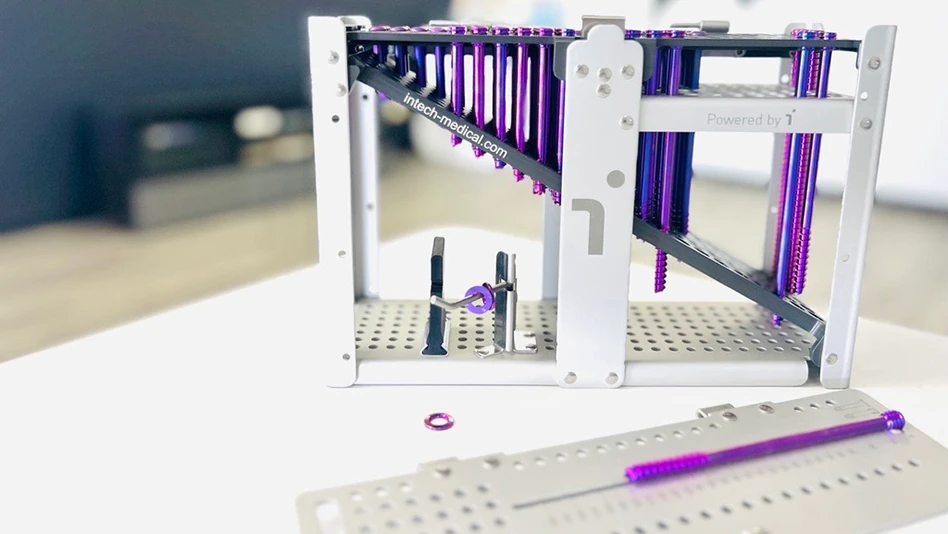
Intech
Intech, the market leader in the manufacturing of orthopedic medical devices, announced that its new Intech Cases Inc. facility in Rockaway, New Jersey, has been awarded ISO13485 certification by AFNOR. Dedicated to the manufacturing of surgical cases, trays, and caddies for the orthopedic industry, this 11,000ft2 expansion allows Intech to further enhance its competitive responsiveness by increasing capacity to meet the growing demand for cases & trays in the United States.
“We are proud of all the hard work this certification represents and demonstrates our quality commitment to our customers,” says David Hollner, plant manager at IntechRockaway. “This certification, along with FDA registration, highlights this facility’s commitment to producing top-notch surgical containers while providing Intech with a platform to better serve its customers on both sides of the Atlantic.”
Premium surgical trays and caddies with lightning-fast delivery
The Rockaway site effectively becomes the US extension to Intech’s 30+ years expertise in cases & trays manufacturing at Saint-Soupplets: a 40,000ft2 facility with robotic press brakes and state-of-the-art automation. The New Jersey site has been taking delivery of its first equipment since January of 2022 and is expected to complete its cleaning validations by end of June 2023 to further expand Intech’s exceptional responsiveness with production of cases & trays within 10-12 weeks.
Latest from Today's Medical Developments
- IMTS 2026 runs Sept. 14-19 at McCormick Place in Chicago, Illinois
- Master Bond’s MasterSil 800Med
- ZEISS celebrates 100 years of advancing innovation in the US
- Teleflex sells acute care and urology businesses for $2.03 billion
- HANNOVER MESSE: Where research and manufacturing meet
- What’s next for the design and manufacturing industry in 2026?
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out





