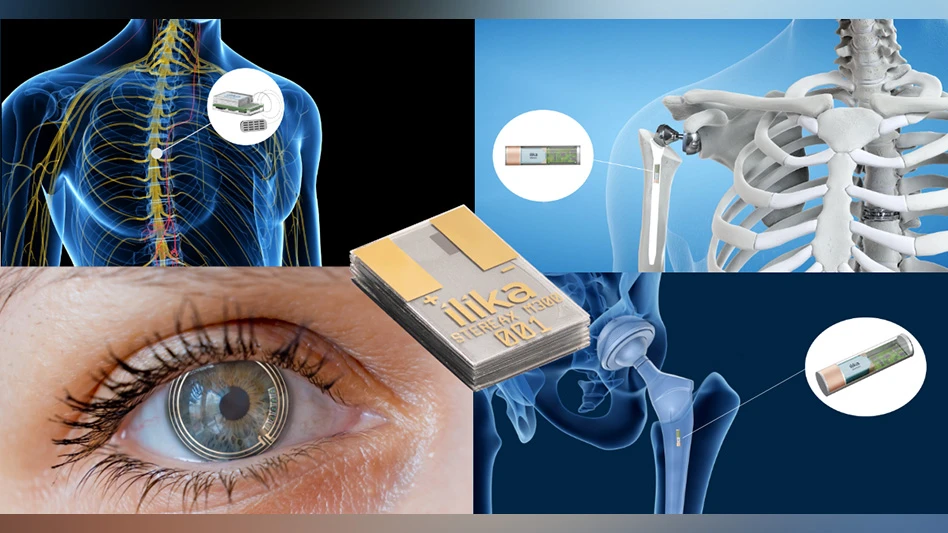
Ilika Technologies
Ilika and Cirtec Medical announce they have concluded contractual negotiations by signing a ten-year manufacturing license to produce the Stereax range of mm-scale batteries at Cirtec's facility in Lowell, Massachusetts, U.S.
Ilika will continue to focus on advanced technology development and IP licensing in support of Cirtec's manufacturing and commercialization activities. This partnership will reinforce Cirtec's ongoing activities in system level miniaturization for the medical device industry.
Graeme Purdy, Ilika's Chief Executive Officer, says: "We are delighted to be working with Cirtec for the commercialization of Stereax. Cirtec has a strong track record in the commercialization of miniature medical devices and we believe this partnership is well-positioned to ensure a high quality, reliable and scalable supply of Stereax batteries to our customers."
Brian Highley, Cirtec Medical Chief Executive Officer, comments: "We are excited by the prospect of adding Stereax battery technology to our portfolio of medical device solutions. Our 20 years of experience in the design, development and manufacture of medical devices positions Cirtec strongly to work with Ilika to bring its Stereax battery technology to market. We believe this collaboration fits right into our strengths and expands upon our mission to vertically integrate our capabilities to support our offerings to the implantable and wearable device markets."
Latest from Today's Medical Developments
- Current economic and geopolitical realities demand decisive action
- Collaboration to advance pediatric medical technologies
- Guyson's TR-1000 Custom Vertical Blast System
- Intelligent data for the digital factory
- Edge Technologies' latest barfeeders
- Arterex expands again with acquisition of Adroit USA
- Teknor Apex material innovations for the healthcare industry
- Archetype appointed to secure EU Market approval for LumipenPro