Ilika
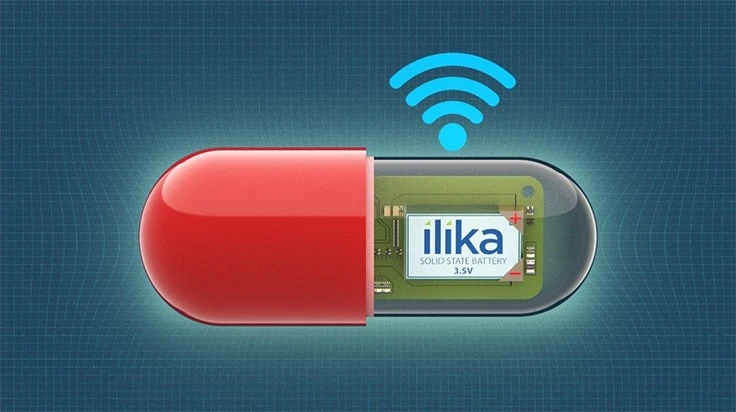
Ilika’s Stereax range of miniature solid state batteries address the challenges of powering next-generation, disruptive medtech devices - both implantable (nerve stimulation, leadless pacemaker or implanted sensors) and small wearables (e.g. smart contact lenses) or hearables. With an ultra-thin form factor, miniature footprint (mm-scale) and customizable in shape and dimensions, they can be stacked for increased energy density whilst remaining safe for implantable devices.
Equipment procurement
In its trading update of July 9, 2020, Ilika confirmed that the contract had been awarded for the most significant long-lead equipment item, Tool 1, a custom-designed evaporation tool for the rapid deposition of cathode materials for Ilika’s Stereax batteries. It is still expected that this will be ready for delivery to Ilika for process development in Q4 2020.
Ilika has also placed the order for Tool 2, which deposits the remaining battery layers on top of the cathode. The delivery of this tool is synchronized with the delivery of Tool 1 to ensure optimal deployment of capital. Tools 1 and 2 are balanced to deliver a 70x productivity increase relative to Ilika’s current pilot line capability.
Third party fabrication facility
Ilika has issued requests for information (RFI’s) to four 3rd party fabrication facilities (FABs), two in Europe and two in North America, to prepare for the selection of its preferred fab partner. Ilika has now entered into detailed commercial discussions with two of the FABs, with a view to concluding discussions in Q4 2020.
Ilika has also appointed Paul Marron to the newly created position of technology transfer director, overssing implementation of Ilika’s process at FAB scale. Marron has extensive operational experience and will bring a wealth of knowledge as Ilika moves to being a manufacturing company.
Ilika’s CEO Graeme Purdy, states, “We remain entirely focused on agile project execution as we prepare our Stereax technology to meet the rigorous demands of full commercialization. We are pleased to report that everything is on schedule, post shareholder support from the recent capital raise, despite the recent challenging economic market conditions.”
Latest from Today's Medical Developments
- North American robotics market holds steady in 2024 amid sectoral variability
- Evident’s DSX2000 digital microscope
- Ferrocene becomes first Rust toolchain to achieve IEC 62304 qualification
- Germany expects a major decline in production in 2025
- Learn what you need to comply with CMMC requirements
- VersaBuilt’s CNC automation possibilities
- ROBOTICS AWARD 2025
- There’s still time to register for this week’s manufacturing industry webinar!