FDA
In response to the March 2019 closure of a large device sterilization facility, the FDA is taking steps to ensure that hospitals, health care providers, and patients have access to medical devices that are safely and effectively sterilized.
The FDA is also sponsoring two public innovation challenges to encourage new developments in medical device sterilization.
- Challenge 1: Identify New Sterilization Methods and Technologies
- Challenge 2: Reduce Ethylene Oxide Emissions
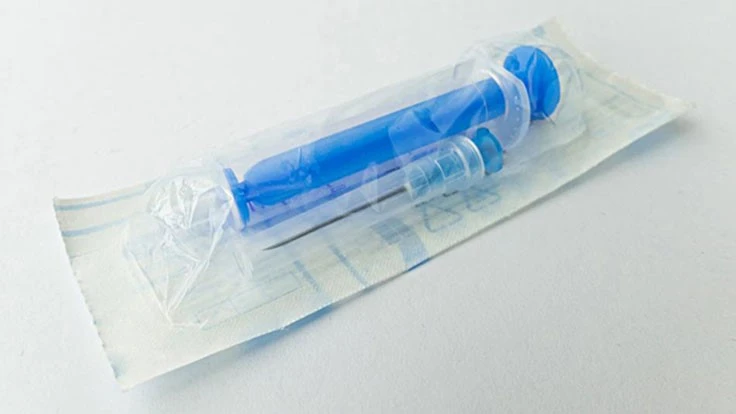
Sterilization is critical in the medical industry. Suzanne B. Schwartz, M.D., M.B.A., Deputy Director of the Office of Strategic Partnerships and Technology Innovation and Acting Office Director, Center for Devices and Radiological Health (CDRH), and CAPT Elizabeth F. Claverie-Williams, M.S., Microbiologist and Branch Chief, Infection Control Devices Branch, CDRH cover the topic of sterilization on the FDA's website in more detail.
They write that, "Before a medical device can be marketed as “sterile,” many medical devices are sterilized by the device manufacturer or contract sterilizer using ethylene oxide before they are delivered to healthcare facilities. Ethylene oxide is a flammable, colorless gas used for equipment and plastic devices that cannot be sterilized by steam. In fact, ethylene oxide sterilization accounts for approximately 50% of medical devices that require sterilization before devices get to patients. And a single sterilization facility can be responsible for sterilizing dozens - or hundreds - of types of medical devices."
Latest from Today's Medical Developments
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out
- Analysis, trends, and forecasts for the future of additive manufacturing
- BlueForge Alliance Webinar Series Part III: Integrate Nationally, Catalyze Locally
- Robot orders accelerate in Q3
- Pro Shrink TubeChiller makes shrink-fit tool holding safer, easier
- Revolutionizing biocompatibility: The role of amnion in next-generation medical devices
- #56 Lunch + Learn Podcast with Techman Robot + AMET Inc.





