LINK Product Development
During the initial and troublesome shortage of personal protective equipment (PPE) that occurred as COVID-19 unfolded in the spring of 2020, our team at LINK found ourselves challenged with designing a reusable N-95 mask for healthcare workers. The goal was aimed at reducing dependency on unstable supply chains and the subsequent shortage of PPE.
What began with a personal connection and passion for lending a hand during a worldwide healthcare crisis, led to a rapid yet thoughtful design project that blended medical device and manufacturing expertise with ingenuity and teamwork.

Opportunity
The opportunity was to design a durable, reusable, and tight-fitting mask surgical mask for frontline healthcare workers as a rapid solution to the critical PPE shortage. Reusability was key, as hospitals and clinics were exhausting supplies of PPE faster than they could be mass produced. Through a series of connections, our team was linked with UC Health, The University of Colorado InWorks program, Make4Covid, and other partners to collaborate on the project.
The initial goal was to develop a mask delivering at least the droplet protection of a surgical mask with a design objective of providing protection against aerosolized viral particles at the N-95 level. From the clinical perspective, the mask needed to be tight-fitting to prevent air from entering other than through the filter to meet the aerosol standard and be comfortable enough to be worn for a prolonged period. According to the stated goal, “The air exchange needed to be adequate for the level of exertion commonly experienced in the clinical environment (ranging from low-intensity sedentary work with the electronic medical record to bursts of high-intensity aerobic exertion during cardiopulmonary resuscitation). It would need to be durable and able to stand up to repeated cycles of decontamination with bleach, alcohol, or disinfectant solution. Finally, the mask design would need to fit within a face shield without excessive fogging. Materials considerations included the need for impermeable materials (other than the filter material itself), biocompatibility, durability, and ability to undergo repeated cycles of disinfection.”

Development of the Livingston Mask
Process
The design process, for what was later coined the Livingston Mask, began by identifying the current challenge or need (lack of reusable N-95 masks for healthcare workers) and important specifications (identified above). This initial discovery process was followed by a series of hand-constructed prototypes and iterations of novel designs.
We began by using silicon molding materials we had on hand to cast an initial mask design within 24 hours of identifying the need. Dissembling other masks used in healthcare settings, experimenting with enhanced versions of the initial design, and casting additional prototypes became the next steps in the process. We used 3D printing to construct the molds required to cast each prototype iteration out of a flexible skin safe thermoset resin.
Once we were connected with a frontline clinician at UC Health, our team was able to test the prototypes in a live setting, secure feedback and turn around an improved design within 24 hours. The rapid-cycle iterative feedback was key in moving the design process forward quickly and with positive improvements. Our team tested a total of seven iterations of the prototype to pass the “qualitative fit test.” The fit test procedure is a requirement put in place by OSHA as a part of a company’s respiratory protection program. This test checks the seal between the respirator face piece and the users face. To detect leakage into the respirator face piece, qualitative fit tests rely on the wearer’s sense of taste or smell as a reaction to an irritant. This is a pass/fail method that relies on whether or not the user detects leakage of the test substance into the face piece. OSHA accepts four qualitative fit test methods:
- Isoamyl acetate, which smells like bananas
- Saccharin, which leaves a sweet taste in your mouth
- Bitrex, which leaves a bitter taste in your mouth
- Irritant smoke, which can cause coughing
We worked through iterations of our prototypes until we consistently passed qualitative fit tests.
Materials
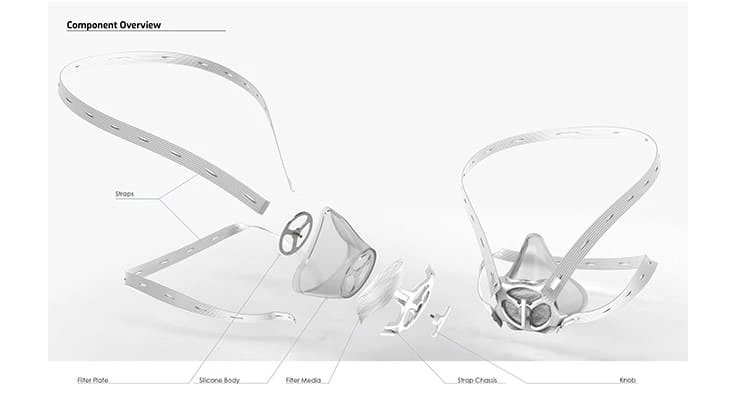
The following components were assembled and used in the initial design of the Livingston Mask.
- Silicon mask chassis
- Top & bottom straps
- Metal disk
- Plastic housing
- Filter material
- Knob

For prototyping, a skin safe product from Smooth-On was utilized. This product is easily mixed and cast using silicone molds. We laser cut and 3D printed plastic components and sourced elastic from Rockywoods Fabrics in Colorado. As the product moved closer to production, we engaged Protolabs in Minnesota to cut tools to injection mold the plastic components. Protolabs also injection molded the mask body in a thermoplastic, medical-grade, flexible material that was compatible with common cleaning solutions used in the hospitals. Focused Light, a laser cutting service company in Denver, provided the metal disks as a core component of the filter assembly. The straps of the facemask were sourced from a variety of vendors in several design iterations in case of supply chain shortages. The design could use basic buttonhole elastic webbing or even rubber bands if necessary. The filter material was, of course, the most difficult item to source on this list. We worked with a supplier that previously made filter material for other industries and had re-tooled their factory to create N-95 comparable material. We worked directly with this source to create custom disks that would work with this mask, using (and hence wasting) much less material than traditional masks that are single use disposable.

Development of the Livingston Mask
Key learnings
The iterative process for the Livingston Mask, which included initial design and tooling to production of samples, was completed in just over four weeks. The development of a new product from concept to production in a month’s time is unprecedented, however, there were factors which could have accelerated the process and are fruitful lessons for future medical device projects.
Without a design team and suppliers, such as Protolabs and Focused Light, who were all willing to work overtime and weekends to expedite parts, the project could not have been executed within the same timeframe. The filter material suppliers were not able to respond as quickly, and project management of these vendors and assimilation into the cause was quite difficult. Relationships with critical supply chain vendors should have been priority one. This is also the case with normal product development cycles, and apparent in today’s global supply chain situation. It was also evident that the more capabilities the product developer has in house, the better. We are fortunate to have a plethora of 3D printers, a laser cutter, molding capabilities, machining, and welding within our studio. The addition of an injection molding machine, a die cutter, and a few other pieces of equipment would have lessened the project’s dependency on external suppliers. Raw materials could be more quickly processed onsite as opposed to by the vendor and design iterations could have been accomplished even faster.
While the testing phase slowed the project past the critical point of need, we were able to navigate vendor and supply chain issues and collaborate with many team members to rapidly develop an open-source design with an injection mold tool, which lays in wait for subsequent waves of COVID-19, future healthcare crisis or impending medical needs.
Latest from Today's Medical Developments
- Machine learning framework enhances precision, efficiency in metal 3D printing
- SkillMill – 60-year-old milling machine with digital twin
- Lumetrics’ OptiGauge II EMS
- EMI completes installation of 128-axis CNC turning & milling machine
- Ottobock invests in innovative technologies from MIT start-ups
- Air Turbine Technology's high-speed live tools for Swiss Lathes
- Sandvik announces several software acquisitions
- Dart Controls’s EZ VFD, variable frequency drives





