Courtesy of the researchers
Implantable devices that
release insulin into the body hold promise as an alternative way to treat
diabetes without insulin injections or cannula insertions. However, one
obstacle that has prevented their use so far is that the immune system attacks
them after implantation, forming a thick layer of scar tissue that blocks
insulin release.
This phenomenon, known as the foreign body response, can also
interfere with many other types of implantable medical devices. However, a team
of MIT engineers and collaborators has now devised a way to overcome this
response. In a study of mice, they showed that when they incorporated
mechanical actuation into a soft robotic device, the device remained functional
for much longer than a typical drug-delivery implant.
The device is repeatedly inflated and deflated for five minutes
every 12 hours, and this mechanical deflection prevents immune cells from
accumulating around the device, the researchers found.
“We’re using this type of motion to extend the lifetime and the
efficacy of these implanted reservoirs that can deliver drugs like insulin, and
we think this platform can be extended beyond this application,” says Ellen
Roche, the Latham Family Career Development Associate Professor of Mechanical
Engineering and a member of MIT’s Institute for Medical Engineering and
Science.
Among other possible applications, the researchers now plan to
see if they can use the device to deliver pancreatic islet cells that could act
as an “bioartificial pancreas” to help treat diabetes.
Roche is the co-senior author of the study, with Eimear Dolan, a
former postdoc in her lab who is now a faculty member at the National
University of Ireland at Galway. Garry Duffy, also a professor at NUI Galway,
is a key collaborator on the work, which appears in Nature Communications.
MIT postdocs William Whyte and Debkalpa Goswami, and visiting scholar Sophie
Wang, are the lead authors of the paper.
Modulating
immune cells
Most patients with type 1 diabetes, and some with type 2
diabetes, have to inject themselves with insulin on a daily basis. Some
patients use wearable insulin pumps that are attached to the skin and deliver
insulin through a tube inserted under the skin, or patches that can deliver
insulin without a tube.
For many years, scientists have been working on
insulin-delivering devices that could be implanted under the skin. However, the
fibrous capsules that form around such devices can lead to device failure
within weeks or months.
Researchers have tried many approaches to prevent this kind of
scar tissue from forming, including local delivery of immunosuppressants. The
MIT team took a different approach that does not require any drugs — instead,
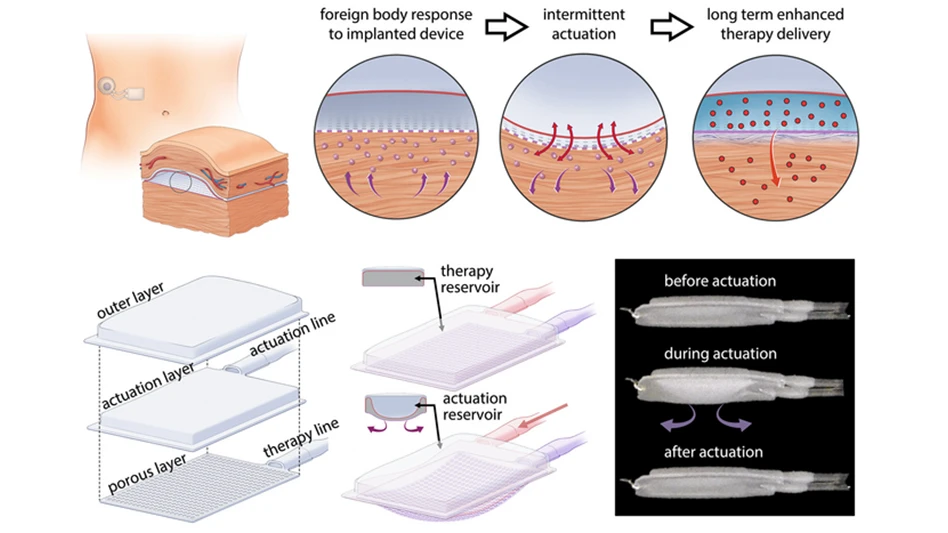
their implant includes a mechanically actuated soft robotic device that can be
inflated and deflated. In a 2019 study, Roche and her colleagues (with
Dolan as first author) showed that this kind of oscillation can modulate how
nearby immune cells respond to an implanted device.
In the new study, the researchers wanted to see if that
immunomodulatory effect could help improve drug delivery. They built a
two-chambered device made of polyurethane, a plastic that has similar
elasticity to the extracellular matrix that surrounds tissues. One of the
chambers acts as a drug reservoir, and the other acts as a soft, inflatable
actuator. Using an external controller, the researchers can stimulate the
actuator to inflate and deflate on a specific schedule. For this study, they
performed the actuation every 12 hours, for five minutes at a time.
This mechanical actuation drives away immune cells called
neutrophils, the cells that initiate the process that leads to scar tissue
formation. When the researchers implanted these devices in mice, they found
that it took much longer for scar tissue to develop around the devices. Scar
tissue did eventually form, but its structure was unusual: Instead of the
tangled collagen fibers that built up around static devices, collagen fibers
surrounding actuated devices were more highly aligned, which the researchers
believe may help drug molecules to pass through the tissue.
“In the short term, we see that there are fewer neutrophils
surrounding the device in the tissue, and then long term, we see that there are
differences in collagen architecture, which may be related to why we have
better drug delivery throughout the eight-week time period,” Wang says.
Sustained
drug delivery
To demonstrate the potential usefulness of this device, the
researchers showed that it could be used to deliver insulin in mice. The device
is designed so that insulin can slowly seep out through pores in the drug
reservoir or be released in a large burst controlled by the actuator.
The researchers evaluated the effectiveness of the insulin
release by measuring subsequent changes in the mice’s blood glucose levels.
They found that in mice with the actuated device, effective insulin delivery
was maintained throughout the eight weeks of the study. However, in mice that
did not receive actuation, delivery efficiency began to wane after only two
weeks, and after eight weeks, almost no insulin was able to pass through the
fibrous capsule.
The authors also created a human-sized version of the device,
120 millimeters by 80 millimeters, and showed that it could be successfully
implanted in the abdomen of a human cadaver.
“This was a proof of concept to show that there is a minimally
invasive surgical technique that could potentially be employed for a
larger-scale, human-scale device,” Goswami says.
Working with Jeffrey Millman of the Washington University School
of Medicine in St. Louis, the researchers now plan to adapt the device so that
it could be used to deliver stem-cell-derived pancreatic cells that would sense
glucose levels and secrete insulin when glucose is too high. Such an implant
could eliminate the need for patients to constantly measure their glucose
levels and inject insulin.
“The idea would be that the cells would be resident in the
reservoir, and they would act as an insulin factory,” Roche says. “They would
detect the levels of glucose in blood and then release insulin according to
what was necessary.”
Other possible applications the researchers have explored for
this kind of device include delivery of immunotherapy to treat ovarian cancer,
and delivering drugs to the heart to prevent heart failure in patients who
have had heart attacks.
“You can imagine that we can apply this technology to anything
that is hindered by a foreign body response or fibrous capsule, and have a
long-term effect,” Roche says. “I think any sort of implantable drug delivery
device could benefit.”
Latest from Today's Medical Developments
- CCAI Finishing Education Foundation’s 2025 National Scholarship Program
- Moticont’s next linear servo motor in the GVCM-032 series
- Walter USA unveils new state-of-the-art campus
- Fixtureworks’ manual-style snap clamps
- Cutting Tool Market Report shows orders up from Sept. 2024
- Mahr’s expanded Precimar SM 60 length measurement family
- Prosthetic material could reduce infections from intravenous catheters
- The Okuma GENOS L3000-e MYW Brings Versatility to the Table