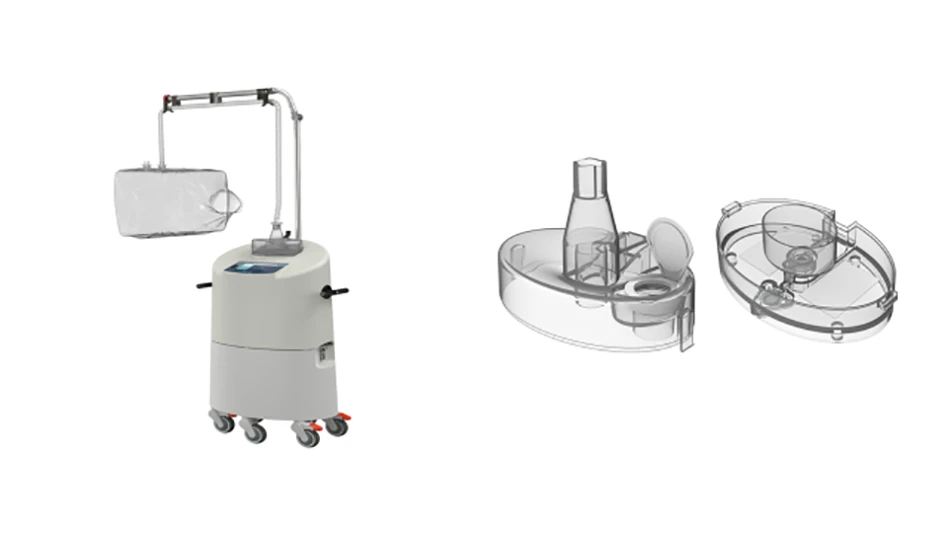
Credit: Covestro & Vaporox
Chronic wounds afflict millions of people. If not treated adequately, they can lead to severe complications, including amputation of limbs. Denver-based Vaporox developed a medical device for healing chronic wounds that do not respond to conventional wound treatment. The vaporizer component of the medical device primarily utilizes Makrolon 2458 polycarbonate from Covestro.
The VHT-200 (Vaporous Hyperoxia Therapy) system, which received U.S. Food and Drug Administration (FDA) clearance, features a unique combination of elements that accelerate healing – ultrasonic vapor and concentrated oxygen. According to Vaporox, the patented delivery system for these elements promotes revascularization and tissue growth. The treatment is intended for administration in clinics and physicians’ offices.
“Vaporox is committed to a world where all wounds heal,” says Chase Huskey, marketing manager, Vaporox. “Today, wound care is one of the most under-served markets in the medical industry, but this development is truly life-changing for patients and a huge step forward for this market.”
Covestro was involved throughout the device’s development, supplying prototype quantities of its material, and documentation required by the FDA.
Makrolon 2458 polycarbonate is a low-viscosity, injection-molding medical grade that features easy release from the mold. It is suitable for Ethylene Oxide (EtO) and steam sterilization and is biocompatible according to many ISO 10993-1 test requirements. It is available in transparent and opaque options.
“For decades, the healthcare market has relied on our proven portfolio of medical grade Makrolon polycarbonates, complemented by customized technical expertise,” says Mark Nichols, healthcare key account manager, Covestro LLC. “Our work with Vaporox demonstrates the value of collaboration as our customers develop and bring truly innovative, game-changing technology to market.
Latest from Today's Medical Developments
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out
- Analysis, trends, and forecasts for the future of additive manufacturing
- BlueForge Alliance Webinar Series Part III: Integrate Nationally, Catalyze Locally
- Robot orders accelerate in Q3
- Pro Shrink TubeChiller makes shrink-fit tool holding safer, easier
- Revolutionizing biocompatibility: The role of amnion in next-generation medical devices
- #56 Lunch + Learn Podcast with Techman Robot + AMET Inc.





