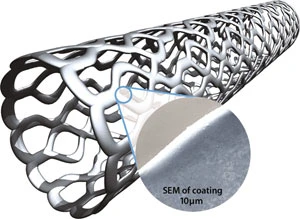
Coatings are consistent within 3% on thickness, with no voids, webs or rough surfaces.
Manufacturers of stents have finally gotten a better handle on one of their primary process-control issues-- accurately applying costly anti-rejection coating to the underlying mesh. The solution that they have discovered is ultrasonic coating.
As a result, that same technology is spreading into other applications of implantables that have comparable strict requirements. Spinoff applications include subcutaneous glucose monitoring sensors, angioplasty guide wires, catheters, and hernia-repair mesh. Depending on the applications, the coatings contain such expensive active ingredients as antibiotics or anti-rejection compounds, biocompatible lubricants or electrolytes, which are entrained in a variety of solvents and polymers.
Around the globe, more than 50 stent manufacturers using ultrasonic coating now report holding coating consistency within 3%, and transfer efficiencies of 30% to 70%. By contrast, older spray processes or dip coating variations average 5% to 7%, and efficiency averages just 7% to 15%.
"These gains are especially important in stent manufacture since elution rate of the active agent depends directly on coating thickness," says Chris Coccio, president of Sono-Tek Corp., supplier of ultrasonic coating systems for the medical device industry. "Higher efficiency means less overspray of a costly ingredient, translating directly into lower operating costs."

Technician coating a stent with Sono-Tek's Medi Coat laboratory scale ultrasonic stent coating system.
The company has hundreds of ultrasonic nozzles at more than 50 medical device companies. About two thirds of the nozzles are built into stent manufacturers' own proprietary coating systems. The others are in the field as part of company's Medi Coat bench-top spray coating system.
Unlike older spray methods, which are difficult to control, ultrasonic atomization uses ver y controllable ultrasonic energy to shatter the liquid stream into uniformly tiny droplets. It creates a soft, low velocity spray of uniformly tiny droplets to produce a very thin, straight spray pattern. Depending on nozzle selection, the spray pattern can be as fine as 0.010 in. wide and repeatable within 3%.
On stents, the finished coating applied ultrasonically is smooth, of uniform thickness, and free of webbing in the mesh. Often, ultrasonic stent coating is done in a nitrogen atmosphere to improve liquid flow characteristics and to reduce surface tension as the droplet contacts the stent surface.
In a typical stent coating process, the stent is mounted on a mandrel which rotates and axially feeds the stent under the ultrasonic spray nozzle in a programmed cycle. The fine spray itself is created by injecting the liquid into a very fine stream of inert gas exiting from the ultrasonic nozzle. Droplet size can be regulated down to a few microns, +/- 3%. The gas flow containing the droplets is 95% slower than in air atomization systems, which accounts for the lower overspray and the closer control over coating placement and thickness. The focused gas stream then transports the spray down onto the stent. Flow rates are set between 20 µl/min and 100 µl/min, and line widths of the spray pattern are in the range 0.020 in. to 0.080 in. (0.5mm to 2.0mm).
It usually takes several passes to build up to a specified thickness with the end point as indicated by weight gain.
The first medical use of ultrasonic spray coating dates back to the 1980s, for coating the insides of blood collection tubes with anticoagulants, preservatives and other reagents. It expanded into stent manufacture around 2001, first with Sono-Tek nozzles going into custom systems. At that time, stent manufacturers were also trying to make older-technology spray, dip coating and other methods work, but they encountered high reject and overspray rates. In 2003, Sono-Tek developed the Medi-Coat "Plug and Play" lab-scale automatic stent coating system, which includes all of the integrated controls, part handlers and utility connections necessary for controlled stent coating. More than 60 Medi-Coat systems are in use, including many multiple orders and reorders for both laboratory work and low-volume production.
Based on success in stent production, variations of Medi-Coat systems are in different stages of validation. The various applications performed by Medi-Coat require the same exacting control as for stents.
Depending on the application, solvents delivered by the ultrasonic method include tetrahydrofuran, chloroform and ethanol, THF, acetone, DMAC, and toluene. Polymers delivered have included urethanes, polyectides, polycarbonates, silicones and styrenes. Active-agent concentrations have ranged from 0.5% to 3%.
Mr. Coccio expects the demand for precision coatings to continue growing among medical device providers. "First, stent implantation continues to grow rapidly worldwide as a medical practice, with new stent providers entering the field regularly. Beyond that, implants, catheterization, and microsurgical and minimally-invasive surgical and diagnostic procedures are on the rise," explains Coccio. "In many cases, their success will hinge on very precise polymer coatings on devices that enter the patient's body," he continued. "And ultrasonic coating is fast becoming the coating method of preference for ultraprecise coating of medical devices."
Anticipating this growth, Sono-Tek is working on a production-scale version of the Medi-Coat system. Slated for introduction in the Spring of 2007, Medi-Coat II will also be Plug-and-Play, and will be capable of coating up to eight stents at a time.
"The bottom line is, with the proven ultrasonic coating technologies and knowledge base available today, there's no need anymore to tolerate high reject rates and waste stemming from the coating process," says Mr. Coccio.

Explore the September 2007 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- HERMES AWARD 2025 – Jury nominates three tech innovations
- Vision Engineering’s EVO Cam HALO
- How to Reduce First Article Inspection Creation Time by 70% to 90% with DISCUS Software
- FANUC America launches new robot tutorial website for all
- Murata Machinery USA’s MT1065EX twin-spindle, CNC turning center
- #40 - Lunch & Learn with Fagor Automation
- Kistler offers service for piezoelectric force sensors and measuring chains
- Creaform’s Pro version of Scan-to-CAD Application Module





