Enhancing Surgical Outcomes, Ensuring Accuracy of Orientation
Enhancing Surgical Outcomes, Ensuring Accuracy of Orientation
Officials from NeuroLogica Corp. and Stryker Navigation, a business unit of Stryker Corp., announce the development of an integrated surgical navigation unit for NeuroLogica’s portable BodyTom 32-slice CT scanners.
The compatibility of both technologies will offer surgeons better orientation in the OR and enable minimally invasive surgeries with higher accuracy. Design of NeuroLogica’s portable BodyTom CT scanners and Stryker’s surgical navigation system platforms are to assist with a wide variety of medical procedures, including spine, trauma, neurosurgery, and ENT. neurologica.com; stryker.com
 FDA 510(k) Submittal for IV Catheter System
FDA 510(k) Submittal for IV Catheter System
Tangent Medical officials announce the submission of a 510(k) for the company’s NovaCath Secure IV Catheter System, developed from extensive clinical research of healthcare worker and patient needs. This closed IV catheter system is a next-generation technology designed to address several complex IV therapy challenges including catheter stabilization, occupational exposure to blood, and patient discomfort. When cleared by the FDA, the NovaCath system will be the first safety IV catheter offering advanced catheter stabilization technology designed to exceed the highest CDC, OSHA, and INS standards for peripheral IV catheter stabilization. tangentmedical.com
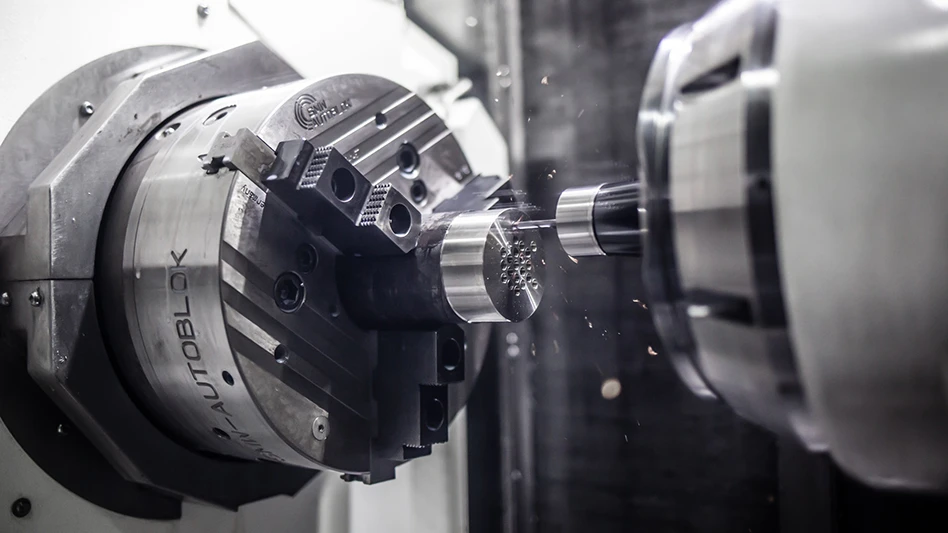
 Order in Excess of $3 Million
Order in Excess of $3 Million
Zygo Corp.’s, Optical Systems Division was awarded an order last month valued at more than $3 million by a major medical device company to produce high precision lens assemblies. Manufacturing of the order will be by Zygo’s Electro-Optics group in its Tucson, AZ, and Costa Mesa, CA, facilities.
“Design and manufacture of complex high precision objective lenses across many markets is one of Zygo’s core competencies,” states John Stack, president of the Optical Systems Division. “Our Electro-Optics group’s FDA registration and strict adherence to ISO 13485 medical device standards, combined with their precision manufacturing capabilities, establish Zygo as a manufacturer of choice for medical device companies seeking proven quality standards.” zygo.com
![]() New Growth Opportunities
New Growth Opportunities
NineSigma’s recent survey showed the leading benefits of open innovation to solution seekers are minimizing risk and accelerating time to market. NineSigma provides services to these organizations every step of the way. NineSigma will roll out a new program in the next two months for members of its dynamic innovation community. Learn more at bit.ly/HxeyOp.
 Bio-Absorbable Magnesium Alloys
Bio-Absorbable Magnesium Alloys
For several years, Magnesium Elektron has been actively working on the development and production of bio-absorbable magnesium alloys at its Magnesium Technology Centre in Manchester, UK. The work has involved extensive research into numerous alloy systems and the development of novel manufacturing technologies. Through collaborative programs, the new Elektron Synermag range of bio-absorbable alloy technologies has already undergone considerable in-vitro and in-vivo evaluations. Plans are for further evaluations and trials during 2012. magnesium-elektron.com
 GCMI Supports Medical Device Companies
GCMI Supports Medical Device Companies
Two Georgia companies will each receive up to $50,000 in convertible notes from the Global Center for Medical Innovation (GCMI) – ICON Interventional Systems and MMJ Labs. ICON Interventional Systems is an emerging medical device company providing cardiovascular solutions to patients and doctors around the world. MMJ Labsdevelops reusable, inexpensive products for personal pain control.
“We are pleased to be able to provide pre-seed money that is so critical to very early stage companies,” says GCMI’s Executive Director, H. Wayne Hodges. icon-us.com; buzzy4shots.com; devices.net
 Regulation Guide Subscription
Regulation Guide Subscription
The “Guide to Medical Device Regulation” is an in-depth analysis on medical device regulations; a practical guide that will aid in making informed and efficient decisions by providing the most up-to-date regulatory developments and guidance on how the FDA is interpreting and enforcing compliance regulations. Stay in compliance through useable, accurate, timely, and comprehensive information on federal regulations and guidance governing the FDA’s approval, investigation, monitoring, and enforcement activities related to devices commercially distributed in or exported from the United States. bit.ly/HoNqk3
 Siemens Hearing Instruments’ five new XCEL models – the new generation of its clinically proven BestSound Technology – accelerate hearing aid acceptance by optimally balancing speech intelligibility and sound quality for each individual hearing aid wearer. usa.siemens.com/hearing
Siemens Hearing Instruments’ five new XCEL models – the new generation of its clinically proven BestSound Technology – accelerate hearing aid acceptance by optimally balancing speech intelligibility and sound quality for each individual hearing aid wearer. usa.siemens.com/hearing

Explore the May 2012 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Robotics action plan for Europe
- Maximize your First Article Inspection efficiency and accuracy
- UPM Additive rebrands to UPM Advanced
- Master Bond’s LED415DC90Med dual-curable adhesive
- Minalex celebrates 60 years of excellence in miniature aluminum extrusions
- Tormach’s Chip Conveyor Kit for the 1500MX CNC Mill
- #39 - Lunch & Learn Podcast with EMUGE-FRANKEN USA and Okuma America
- Significant expansion for Intricon