Vertebral compression fractures are one of the most common types of spinal injuries. More than 700,000 Americans experience vertebral fractures every year, with approximately 500,000 of those caused by osteoporosis, the rest by trauma or injury.
With the Baby Boomer generation nearing retirement age, and life expectancy figures continuing to rise, the incidence of osteoporosis-related vertebral fractures is only expected to increase as the population ages.
Compression fractures cause vertebrae to collapse partially, causing the spinal column above the damaged bone to bend forward. Vertebral compression fractures cause pain that ranges from mildly irritating to incapacitating, and can lead to loss of height, immobility, deformity, and in some cases, reduced pulmonary function. At their most severe, vertebral fractures profoundly affect quality of life and self-esteem, yet, it is documented that as many as 45% of vertebral fractures go undiagnosed, and many more are untreated. The market for surgical tools used to repair these fractures has been estimated at $570 million.
SURGICAL OPTIONS
Vertebroplasty, used in the United States since 1991, uses a needle guided by X-rays to inject a small quantity of a liquid polymer, polymethylmethacrylate (PMMA), into the damaged bone.
PMMA fills the cracks and crevices of the fracture.
As it reaches body temperature, the PMMA hardens, keeping the fractured bone from moving and eliminating pain from the movement impinging the nerves.
A newer procedure, kyphoplasty, has been available since 2002. Kyphoplasty is somewhat more complex than vertebroplasty, but it also involves injection of PMMA into the damaged bone.
The primary differences are that vertebroplasty injects PMMA into the fracture and allows the cement to creep into the crack and crevices of the fracture. Kyphoplasty uses a balloon to push the soft spongy cancellous bone into the crack and crevices stabilizing the fracture and then filling the void created by the pushing from the center with the PMMA.
Kyphoplasty is conducted with the guidance of imaging X-rays and a contrast medium. In this procedure, two small incisions are made in the skin directly over the affected vertebra.
Drills are inserted through a surgical probe deep into the bone and retracted through the probe. The probe serves as a working channel.
Two bone tamps are then inserted through the probes and inflated. The action of the balloons expanding pushes the soft bone tissue inside the vertebra outward and into the fracture, restoring the vertebra to its natural dimensions.
After the balloons have reached the desired volume they are deflated and the bone tamps are removed.
The empty volume is then filled with liquid PMMA through two needles which are retracted as the cavity fills. The PMMA hardens rapidly when it reaches body temperature, filling the volume formerly occupied by the balloon volume.
Kyphoplasty has a number of benefits, among them short surgical time, rapid recovery, hospital stays of usually one day or less, and no bracing required after surgery. However, Kyphoplasty's principal flaw is its reliance on two probes and two points of intervention for one damaged vertebra. The introduction of two probes practically doubles the likelihood of complications such as infection or damage to sensitive spinal nerve bundles.
When PMMA is introduced during kyphoplasty, the polymer fills two separate voids in the bone that are frequently of uneven and unequal dimensions.
X-rays of treated vertebrae frequently show leakage of PMMA outside the treated area, which is believed to lead to an unacceptably higher risk for subsequent vertebral fractures in adjacent vertebrae.
During the balloon inflation stage of kyphoplasty soft vertebral bone, known as cancellous bone, is pushed to the periphery of the cavity formed by the balloons, thereby preventing PMMA from completely infiltrating the outer areas of the fracture. This causes a less-thanideal sealing of the fracture. At the same time, once the balloons are withdrawn the cavities are poorly defined. This leads to an inability to control the application and distribution of PMMA, and diminishes its structural integrity once it hardens.
So-called stress risers result, causing pressure from above the fracture to be unevenly distributed onto the supporting bone below.
While kyphoplasty claims to restore height, the clinical data shows otherwise. The U.S. Food and Drug Administration has therefore asked that Kyphon Inc., the developer of kyphoplasty, withdraw its claim that this procedure restores height.
 |
 |
 |
 |
SIMPLER MAY BE BETTER
Although it has been hailed as the second-generation surgical option for vertebral compression fractures, kyphoplasty has fallen short of producing tangible benefits compared with vertebroplasty. Since 2007, a new option has been available that provides what older procedures have promised but not delivered.
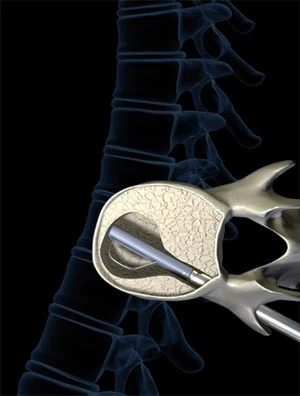
Ascendx, now in clinical testing, is a novel approach to treating vertebral fractures. In this procedure one surgical drill is inserted through a working probe, via a single incision above the affected vertebra, to create a channel through to the soft inner bone. After withdrawing the drill, a second device, called an articulating cutting device, is inserted to create the void into which PMMA will be applied.
The cutting device, contained in a single pedicle, has two features that distinguish it from the balloon device that creates the cavity during kyphoplasty. The first is a retractable blade located within the tip of the pedicle. This blade carves out a single large cavity within the vertebra by cutting the cancellous bone.
The second advantage is that the tip bends sideways as it rotates to access as much of the cancellous bone as possible. In the process, it creates a single large volume inside the vertebra instead of two smaller volumes. Once a cavity of sufficient volume is created, the tip straightens, the blade retracts, and the cutting device is removed.
The next probe inserted, the fracture reduction device, also has a dual purpose. Like the one used in kyphoplasty it contains an inflatable mechanical jack that pushes on the hard parts of the bone putting it back to its proper shape. This happens within the cavity formed by the articulating cutting device. After the mechanical jack (reduction device) inflates, liquid PMMA is injected through a tube running alongside the reduction device.
The process of filling begins while the balloon is fully or nearly fully inflated and occurs from the outside of the balloon. As the cavity fills with cement, the balloon is simultaneously deflated. Once the cavity is filled, the balloon reduction probe is retracted.
BENEFITS OF ASCENDX Because Ascendx is an investigational device, clinical outcomes are still being evaluated. Ascendx, however, appears to show potential numerous advantages over the standard of surgical care for vertebral compression fractures. Ascendx seems simpler and streamlined by virtue of operating within one operating channel instead of two. Vertebral entry points are halved, which reduces trauma to the vertebra and the likelihood of adverse events such as spinal nerve bundle complications.
Most significantly, Ascendx creates a single, large cavity from the soft bone instead of two smaller cavities. This seems to result in uniform distribution of PMMA in the cavity that may suggest superior post-surgical force load distribution.
This is perhaps best illustrated by the analogy of an automobile jack. When changing a tire the jack is positioned under a bracket connected to the frame of the car. The car's weight is distributed within the frame instead of at a single point.
With respect to vertebral fractures, this phenomenon may mean the weight of the patient's upper body, which is supported by the spine, is evenly distributed across the entire repaired vertebra and those below, instead of at two smaller points (the case with kyphoplasty). This in turn would put less stress on adjacent vertebrae and possibly lessen the likelihood that they will also fracture.
Uniform force load distribution would be particularly beneficial for patients with brittle spines caused by osteoporosis.
AOI Medical
Orlando, FL
aoimedical.net

Explore the August 2009 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out
- Analysis, trends, and forecasts for the future of additive manufacturing
- BlueForge Alliance Webinar Series Part III: Integrate Nationally, Catalyze Locally
- Robot orders accelerate in Q3
- Pro Shrink TubeChiller makes shrink-fit tool holding safer, easier
- Revolutionizing biocompatibility: The role of amnion in next-generation medical devices
- #56 Lunch + Learn Podcast with Techman Robot + AMET Inc.





