Medical adhesive tapes designed to adhere to a patient’s skin have a reputation for being one of the most complex to formulate.
First, they need to stick to the skin, which is a multi-layered, living, and fluid system constantly passing moisture, excreting oils, shedding the surface layer of cells, and more. Tapes need to stay in place after they’re applied and meet intended wear times, withstanding environmental factors such as temperature changes and exposure to moisture. Because of the skin’s ability to experience pain, comfort for the wearer is also key, calling for attributes such as gentle removability, conformability, and breathability. Adhesive tape formulations for skin applications must be highly specialized. But are skin tapes the most complex in the market?
To the surprise of many, another tape holds that title. Diagnostic adhesive tapes used in the construction or assembly of in-vitro diagnostics (IVD) devices are extremely sensitive and even more complex than skin tapes.
The IVD device market segment is incredibly large. According to Statista data, the total IVD market in 2021 was $83.4 billion and is projected to reach $108.8 billion this year.1 The global point of care diagnostics market is expected to grow from $36.37 billion in 2022 to $51.94 billion by 2029, according to Fortune Business Insights.2 In comparison, Grand View Research reports the global wound care market size was valued at just $21.4 billion in 2022.3
For medical converters, the IVD device market offers a world of opportunity, but it’s often overlooked. Unlike the skin market where applications are common in daily life, diagnostic devices aren’t top of mind. Less than 5 of approximately 80 pressure-sensitive adhesive (PSA) manufacturers specialize in the development of diagnostic adhesives.
A closer look at diagnostics devices
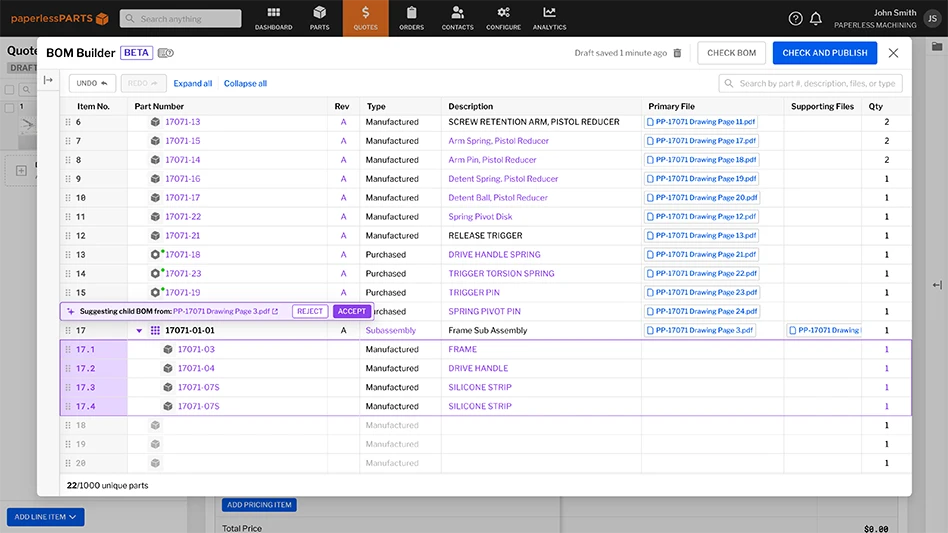
IVD devices are designed to help detect, monitor, treat, and prevent medical conditions or diseases by analyzing body samples such as blood, saliva, urine, or tissue. They’re used in a wide range of at-home, laboratory, and medical facility settings. Examples include lateral flow assay devices such as pregnancy and COVID-19 testing; microfluidic devices testing blood glucose and other blood components; microarray devices for genetic analysis; polymerase chain reaction (PCR) devices for DNA amplification; microtiter plate devices used in drug discovery; and microwells for microbial and pathogen detection.
Even though most are relatively small, diagnostic devices are immensely intricate and feature multi-layer constructions. A “simple” blood glucose strip may consist of 10 or more layers. The most challenging aspect of IVD devices is they bring the body’s chemistries outside of the body for analysis. With the skin no longer protecting these delicate chemistries, there’s a high risk of exposure to environmental elements. Devices must be incredibly clean to ensure accuracy. Any residual monomer, cross-linker, reactive sites, or acid or vinyl functionality could interfere with device performance, making diagnostic adhesives the cleanest – and most complex – adhesives used in medical tapes.
The cleanest of clean
To achieve necessary purity levels, ideal IVD device adhesives are pure acrylic or silicone-based. They have no outgassing, volatile organic compounds (VOCs), or adhesive component migration, and are as inert as possible with little to no residual chemical activity. Currently, most adhesives used in IVD devices are coated from solvent, which poses challenges with residual VOCs, outgassing, and migration.
Water-based adhesives contain surfactants, wetting and defoaming agents, antimicrobial compounds, and more, preventing these adhesives from meeting even the least stringent of cleanliness requirements for diagnostic devices. Rubber-based adhesives are suitable in a few applications, but the large degree of unsaturation and antioxidants often interfere with the detection methods IVD devices use.
One-hundred percent solids adhesive coating is the only method designed to produce an ultra-clean adhesive while achieving the intended bond of device components. When 100% of solids are coated, adhesives are inert, non-outgassing, non-fluorescing, non-migratory, and usable in applications requiring precise deposition control.

Diagnostic adhesive requirements
Although each type of diagnostic device is unique in its workings, they share many similarities in adhesive requirements.
Diagnostic adhesives must maintain chemical stability and adhesion integrity. They often undergo exposure to acids, bases, buffer solutions, alcohols, reagents, and elevated temperatures. In many devices, the adhesive is used to seal wells or channels containing fluids. Adhesive bonds failure can lead to leakages and/or cross-contamination, compromising device functionality.
It’s also important that diagnostic adhesives don’t cold-flow. For example, microfluidic devices transport small amounts of biological fluids through small device channels. Often, the flow of fluids relies on capillary action alone. If the adhesive has excessive creep, channels can become occluded and restrict capillary flow, leading to potential device failure.
Measuring diagnostic adhesive performance accurately and repeatedly is relatively straightforward using analytical techniques. Adhesion testing for diagnostic tapes is also easily performed. Samples are placed in an Instron and peel force is accurately and reproducibly reported against plastic materials used in IVD devices, such as polyethylene terephthalate (PET), polystyrene (PS), polycarbonates (PC), or acrylonitrile butadiene styrene (ABS).
Converting is crucial
Diagnostic tape constructions include transfer tapes, double-coated PET, and single-coated PET. Tapes and adhesives are fabricated in large rolls, requiring a precision converter to transform them into functional parts using processes such as high-speed rotary die-cutting, narrow web platen cutting, laser cutting, slitting, and laminating.
Converting is crucial in IVD device applications – specifically deposition control and precise die-cutting and laminating. Instead of inches, diagnostic applications run at microns, necessitating immense precision. Devices such as diabetic test strips or home pregnancy, microfluidic, and COVID-19 tests with very small, intricate parts require extremely tight tolerances to ensure proper functionality. Thickness and coat-weight control are vital. A blood glucose test strip, for example, is highly calibrated for a very specific and very small amount of blood (microliters). The thickness of the PSA and the precision of die-cuts control how much blood the strip absorbs. Too much blood will show glucose levels that are too high and could result in too much insulin being administered.
Diagnostic adhesive converters need to ensure high levels of cleanliness through ISO-certified cleanroom processing, meeting the strict standards and regulatory requirements for low particulate, temperature, and humidity-controlled die-cutting.
Get curated news on YOUR industry.
Enter your email to receive our newsletters.

Explore the September 2023 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- North America's supply chains face sharp decline due to tariffs
- Experience precision: GF Machining Solutions' CUT F Series wire EDM
- Mastering high-temp alloys with Kennametal Inc.
- Integer expands operations in Salem, creating 83 jobs
- Siemens unveils new Teamcenter X: Revolutionizing SaaS PLM for all manufacturers
- 3 Questions with an Expert with Allied Machine & Engineering
- Supply Chain Power – A strategic program for executives
- Sunnen Products' PGE-6000 gage