Two words best encompass a challenge often faced by medical device designers and manufacturers: speed and reliability.
Intense competitive pressures, combined with demands from the medical community for solutions to combat the spread of disease, have created a marketplace in which even months can be too long for companies to react and produce relevant products.
And yet, speed-to-market must be achieved without compromising the critical need for medical products.
Symbient Product Development strives to achieve both speed and reliability by blending current science and engineering theory, with solid design principles, for the creation of medical device and diagnostic products. In that process, our ability to evaluate a design and anticipate product performance is often highly dependent upon the quality and comprehensiveness of prototyping technology.
Product Challenge
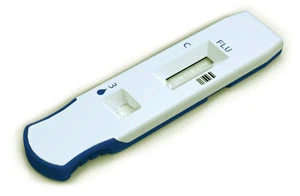
Symbient Product Development was given the opportunity to participate in a fast-timeline project to develop a Diagnostic Lateral Flow Cartridge.
This device uses a lateral flow test strip to evaluate, within a matter of minutes at the point-of-care, whether or not a patient is infected with a virus. In conducting such a test, a fluid sample is collected from the patient and mixed with a buffer, after which several drops of the combined sample and buffer are added to a sample well. The sample is applied, test strips run are run, and a clinician then places the Diagnostic Lateral Flow Cartridge into an instrument that reads the test result.
The design assignment, in parallel with the development of the internal cartridge features, was to develop an exterior industrial design that includes the user interface, features to aid in handling and insertion into the instrument, and aesthetic factors intended to give the product a unique appearance.
In accepting this product design project there was a committment to completing the project within four months - an extremely fast turnaround - without sacrificing the quality of prototyping evaluations that would assure the enduser that the Diagnostic Lateral Flow Cartridge would perform as required.
The first goal was to ensure that our vision aligned with our client's vision and expectations. This required not only input from designers, engineers and administrators, but also the experience of DSM Somos. We knew, from prior experience, that DSM's rapid prototyping materials would provide us with the ability to shorten development time and facilitate the production of functional product models.
Develop, implement the Plan
At the heart of the design and developmental plan was the determination to use WaterShed XC 11122, an optically-clear rapid prototyping resin developed by DSM Somos to provide ABS-like properties and good temperature resistance. WaterShed XC 11122 is a fast-curing, low-viscosity resin, that produces clear, functional, accurate parts that simulate acrylic in appearance, and provide improved water resistance versus alternative resins.
By using stereolithography, several iterations of the Diagnostic Lateral Flow Cartridge design were produced while fine-tuning the geometry necessary to optimize medical assay performance.
These iterations were quickly but accurately evaluated to establish precise parameters for the final design.
The implementation of the plan revolved around short prototyping cycles that were usually characterized by the ability to fabricate prototypes via stereolithography in a single day, followed by one to two days of testing.
This then lead into the design and fabrication of the next iteration on the fourth day.
This design project was unique in that two types of engineering methods, in a parallel path, were employed to complete the device. On one industrial design was utilized. This is the aspect of design that makes the product appealing to the user and includes human factors, branding and ergonomics.
In this case, industrial design involved the grip features at the end of the device that enable a user to hold it in a specific way for proper insertion into the instrument that reads the test result. The pointed end is another industrial design cue that points the user to correctly install the device.
The industrial design also involved a two-color design so that it is visually appealing to users. The overall size and shape are intended to be ergonomic, easy-to-handle and clear-toread.
A variety of industrial design blanks were made, produced through stereolithography, for the customer to evaluate. Based on their feedback, iterations of the industrial design were made, as necessary, until achieving the final design.
On the parallel path, a mechanical design was developed that included features that assemble the product and ensure that the device functions properly. Mechanical design requires a solid understanding of core concepts including fluid mechanics. WaterShed XC 11122 prototyping material allowed visualization of the flow through the strip as it ran during this mechanical design phase.
Additionally, designed and built inside the cartridge were several features that engaged the strip in key areas. The degree of compression was critical for proper strip function; therefore, accuracy and repeatability of the internal cartridge features were extremely important. The mechanical stereolithography models produced delivered the parts needed, and were as close to actual molded components as one could possibly get.
Once the final industrial and mechanical designs were achieved, they were merged into an integrated design that could then be used to create a prototype mold. The fact that stereolithography was able to be used for both mechanical design and for a parallel industrial design was critical to the need for a rapid turnaround.
In order to appreciate how much the stereolithography process and WaterShed XC 11122 contributed to the success of this project, it is important to note that some prototyping iterations required a tolerance of 0.002".
Moreover, this process and material enabled reaching a desired result at a significantly lower cost than possible with machined prototypes. All of this is made more impressive by the fact that we met our obligation of completing this project in less than four months.
Speed and reliability are two challenges successfully met through a balance of proper design and highlyfunctional prototyping materials.
Symbient Product Development
Vista, CA
symbientpd.com
DSM Somos
Elgin, IL
dsmsomos.com

Explore the October 2009 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Blueshift’s AeroZero
- November USMTO grow from October
- Platinum Tooling’s custom and special tooling
- Top 5 global robotics trends 2025
- Accumold’s micro molding innovations
- Methods Machine Tools, Multiaxis, announce AI solution investment
- MGS to showcase global expansion and healthcare CDMO expertise
- Medtronic: 5 healthcare tech trends for 2025





