Through recent advances in its Tactilus plantar pressure measurement technology, Sensor Products LLC is now positioned to assist podiatrists, orthopedists, chiropractors and other medical professionals in making the best diagnoses and recommendations for their patients. Essentially, the emergence of technologies like the Tactilus foot platform and insole systems is critical to the well being of millions of people.
For instance, diabetes is the fifth major cause of disease related deaths in the United States. Of the estimated 18.2 million Americans affected, 4.5 million will suffer from plantar problems like degenerative foot disorder and complications due to neuropathy such as bunions, hammertoes, Charcot feet and more. Experts on the disease stress comprehensive foot care for amputation prevention and overall comfort for diabetes sufferers. Orthotic devices and appropriate footwear play a large role in the quality of life for these patients.

However, diabetics arent the only demographic subjected to pain caused by plantar pressure abnormalities. In fact, about 100 million Americans experience biomechanical plantar problems caused by obesity or arthritis induced heel pain, heel spurs, hammertoes or neuromas. By utilizing Tactilus foot platform and insole electronic pressure measurement sensors, professionals can easily detect, diagnose and monitor patients with these and other plantar abnormalities.
These new analysis systems are the most advanced in the world and can be dedicated to innumerable applications, said Carlos Ruiz, Tactilus product manager. The link between technological advancement and human quality of life is ultimately exhibited because of the diverse global populations the Tactilus systems can potentially benefit.
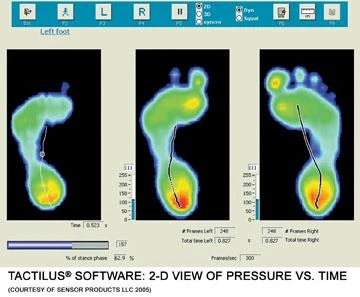
Employing userfriendly software, the foot platform and insole sensor systems generate indepth statistical information and dynamic 2D and 3D profile images for such medical and ergonomic body mapping needs as diabetic and neuropathic patient screening, orthotic and prosthetic efficacy profiling, pronation/supination impact evaluation during bipedal locomotion activities, pre and postsurgical comparative analysis, ulceration detection, degenerative foot disorder monitoring, ray hypermobility diagnosis, and early scoliosis detection. With unprecedented speed and accuracy, the systems comprehensively profile plantar pressure distribution and magnitude.

The Tactilus foot platform analysis system enables professionals to perform dynamic pressure profiling in order to evaluate shoetoground interaction related to the diabetic foot, pronation, foot arch and weightbearing capabilities and assess impact effects in bipedal locomotion activities of both feet, either exclusively, or in relation to each other. The platform detects body motion (footkneehip) to effectively profile any abnormalities. In addition, densely packed sensors in the Tactilus platform analysis system offer the user high resolution images and a modular architecture.
The Tactilus insole systems are advantageous in their basic design by assessing foottoshoe interaction. The foot insole is comprised a thin and highly durable substrate material and ranges in size from unisex US 1.5 to 10.5 (EUR 33 to 44). The insole sensor collects precise data for determining pedal pressure points and assessing athletic plantar implants in activities ranging from standing and walking to running, jumping, skiing and skating. Working at speeds up to 500 Hz, this systems velocity is unmatched by any other technology.
Both the Tactilus foot platform and insole analysis systems possess robust sensors which can endure thousands of uses with consistent repeatability, and are highly resistant to electromagnetic noise, temperature, and humidity fluctuations. The fullscale Windowsbased software provides isobar and regionofinterest viewing, graphical displays of data in bar charts, line scans and histograms, statistical analysis of average/minimum/maximum pressures, total force over any selected area, analysis view of all nine major foot zones, pressure vs. time and more.
Demonstrations of Tactilus analysis systems can be requested by contacting Sensor Products at 1.973.884.1755, tactilussensorprod.com or at www.sensorprod.com/tactilus.
Technical Contact:
Carlos Ruiz
Product Manager
1.973.884.1755 x5001
cruizsensorprod.com

Explore the May 2005 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Robotics action plan for Europe
- Maximize your First Article Inspection efficiency and accuracy
- UPM Additive rebrands to UPM Advanced
- Master Bond’s LED415DC90Med dual-curable adhesive
- Minalex celebrates 60 years of excellence in miniature aluminum extrusions
- Tormach’s Chip Conveyor Kit for the 1500MX CNC Mill
- #39 - Lunch & Learn Podcast with EMUGE-FRANKEN USA and Okuma America
- Significant expansion for Intricon