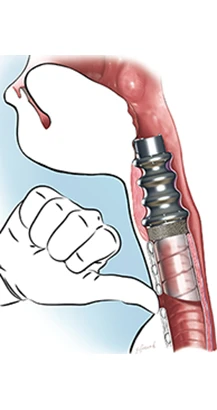
ProTip SAS, the specialist developer of innovative medical devices for patients suffering from laryngeal malfunction, and Strasbourg University Hospitals announce that they have successfully completed the first implantation of an artificial larynx in a human patient.
In June 2012, Professor Christian Debry and his team in the ENT department at Strasbourg University Hospitals carried out the first major surgical procedure on a 65-year-old male with laryngeal cancer.
This first phase of surgery saw the surgical team remove the patient's larynx and implant the first component of the artificial larynx, a tracheal ring made of titanium.
“The main purpose of the tracheal ring that we fitted was to recreate the connection normally formed by the larynx and acting as a kind of funnel between the two,” Debry says. “The material used for the ring means that it can be incorporated into the surrounding tissues, forming an integral part of the throat as a result.”
The second stage in fitting the artificial larynx was completed a few months later, in November 2012. Under general anesthetic, a removable, valve-based device was inserted into the tracheal ring through the patient’s mouth. The artificial larynx device partially replicates the natural functions of the larynx and the patient is again able to breathe through the upper respiratory tract.
Twenty Years of Research for a World First
For Debry, this operation marks the culmination of more than 20 years of research which began as part of his doctoral thesis while he was still a student. Following years of development work in conjunction with Inserm (Institut national de la sante et de la recherche medicale, France's national institute for health and medical research), with the aim of finding suitable biomaterials for replacing the larynx, Professor Debry's idea finally took shape with the selection of a combination of solid and porous titanium, as biological materials with all the properties needed to create the artificial larynx.
ProTip was founded in 2005 with the aim of accelerating the development of innovative medical devices to treat laryngeal pathologies.
“Laryngectomy is a procedure which has not changed in 140 years,” says Maurice Berenger, CEO of ProTip. “This surgical first paves the way for a procedure which gives new hope to laryngeal cancer patients. Ultimately, it will help patients regain their ability to breathe, speak and eat normally.”
Following the promising initial results achieved in the first patient, ProTip and the Strasbourg University Hospitals will continue to develop the technology and the surgical procedure before making the therapy more widely available. To this end, a Europe-wide clinical study is currently underway.
New Hope for Patients with Laryngeal Cancer
“Following a total laryngectomy, patients experience a host of difficulties in re-establishing a normal working and family life, specifically because of the tracheostomy,” Debry states. “My primary motivation in developing this artificial larynx is to offer patients an improved quality of life following this type of procedure. This first operation in Strasbourg is simply the initial feasibility stage. We still have to undertake a number of clinical tests before the larynx implant becomes a routine procedure.”
Source: ProTip Medical
Latest from Today's Medical Developments
- Children’s National, FDA collaborate to advance pediatric device regulatory tools
- LK Metrology’s eco-friendliness CMMs
- Two patents for microfluidic valves
- AMADA WELD TECH’s blue diode laser technology
- Post-IMTS decline in manufacturing technology orders blunted
- ARS Automation’s FlexiBowl 200
- LMA Consulting urges businesses to restructure supply chains now
- Walter’s WEP01C indexable inserts





