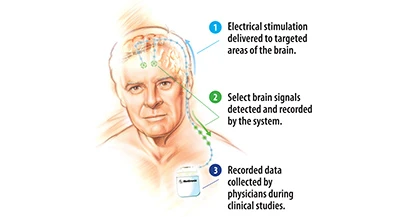
Medtronic Inc. officials announce the first implant of a novel deep brain stimulation (DBS) system that, for the first time, enables the sensing and recording of select brain activity while simultaneously providing targeted DBS therapy. This initiates research on how the brain responds to the therapy and could yield insights that one day significantly change the way people with devastating neurological and psychological disorders, such as Parkinson's disease, essential tremor, dystonia, and treatment-resistant obsessive-compulsive disorder, are treated.
The Activa PC+S DBS system delivers proven Medtronic DBS Therapy while at the same time sensing and recording electrical activity in key areas of the brain using sensing technology and an adjustable algorithm, which enable the system to gather brain signals at various moments as selected by a physician. Initially, this new technology will be made available to a select group of physicians worldwide for use in clinical studies. These physicians will use the system to map the brain's responses to Medtronic DBS Therapy and explore applications for the therapy across a range of neurological and psychological conditions.
The Activa PC+S system, which delivers stimulation to targeted areas of the brain like existing Medtronic DBS systems, was implanted for the first time at Ludwig Maximilians University in Munich, Germany in a person with Parkinson's disease. This patient will be treated by a team that includes neurologist Kai Bötzel, department of neurology, Ludwig Maximilian University, and neurosurgeon Jan Mehrkens, M.D., head of functional neurosurgery, Ludwig Maximilian University, who implanted the system.
Dr. Bötzel will be the first to use data gathered by the Activa PC+S system to gain unprecedented insight into how the brain responds to DBS therapy.
"DBS therapy works for people with Parkinson's disease and other movement disorders, but there is much to learn about how the brain responds to the therapy," says Dr. Bötzel. "This new system will allow us to treat patients with conventional DBS therapy, while at the same time opening the door for research that was not possible until now. We hope these insights will lead to the development of effective new treatments tailored to the needs of individuals."
"Devastating conditions like Parkinson's disease and obsessive-compulsive disorder take a significant toll on countless people, as well as their loved ones," states Lothar Krinke, Ph.D., vice president and general manager of the Deep Brain Stimulation business in Medtronic's Neuromodulation division. "Medtronic is excited to provide this new system to researchers worldwide, and we expect that their respective studies will lead to accelerated understanding of how neurological and psychological conditions develop and progress. This represents a significant milestone for DBS therapy and the long-term journey toward a closed-loop DBS system, which could personalize therapy by using device data to automatically adjust to the needs of individual patients."
Medtronic's Activa PC+S system received CE (Conformité Européenne) mark in January 2013. It is not approved by the U.S. Food and Drug Administration for commercial use in the United States, and will be made available to select physicians for investigational use only. Additional implants of the Activa PC+S system, including the first implant in the United States, will take place in the coming months.
About Medtronic DBS Therapy
DBS therapy uses a surgically implanted medical device, similar to a pacemaker, to deliver mild electrical pulses to precisely targeted areas of the brain. The stimulation can be programmed and adjusted non-invasively by a trained clinician to maximize symptom control and minimize side effects. More than 100,000 patients worldwide have received Medtronic DBS Therapy.
The therapy is currently approved in many locations around the world, including Europe and the United States, for the treatment of the disabling symptoms of essential tremor, advanced Parkinson's disease and chronic intractable primary dystonia, for which approval in the United States is under a Humanitarian Device Exemption (HDE). In Europe, Canada, and Australia, DBS therapy is approved for the treatment of refractory epilepsy. DBS therapy is also approved for the treatment of severe, treatment-resistant obsessive-compulsive disorder in the European Union and Australia, and in the United States under an HDE.
Latest from Today's Medical Developments
- Fed’s soft landing may ignite manufacturing technology market growth
- Platinum Tooling named North American distributor for Dunner
- Bridging the Skills Gap: A Solution for Today’s Labor Shortage
- Machine Solutions acquires Alpine Laser LLC
- OSG USA’s PHOENIX PFDC indexable face mill cutter & inserts
- IMTS 2024 Booth Tour: Fagor Automation Corp.
- How Robotics and Automation are Transforming Manufacturing
- Quasar Medical acquires Ridgeback Technologies