VivaLNK

Healthcare is increasingly moving toward remote patient monitoring (RPM) and telemedicine in order to reduce costs while making care more accessible. This means healthcare developers are looking for solutions combining accurate sensors and quality data to make home healthcare as efficient and productive as possible.
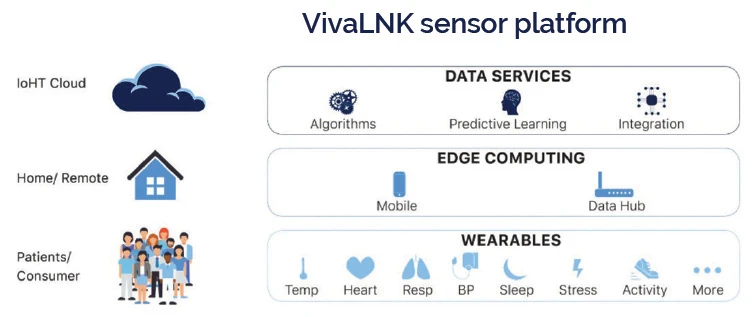
Now, there’s an exciting evolution in medical wearable sensors and platforms that is allowing for more impactful applications of RPM. The emergence of a new category of wearable medical-grade devices, and significantly more accessible cloud platforms to build RPM solutions on, is taking RPM past virtual appointments and patient-reported data. One indicator this evolution has started is the convergence of traditional consumer technology companies into the medical device space, such as Apple with its latest FDA-cleared ECG Watch, allowing for higher quality monitoring available directly to consumers. The first catalyst of this evolution are new sensors available to medical device manufacturers and original equipment manufacturers (OEMs). These new medical devices merge the historically distinct markets of regulated medical devices and consumer wearables. Three key attributes allow these devices to gain more traction in the market: medical-grade data, user-friendly devices, and affordability. These newly enhanced devices overcome healthcare wearability challenges by using form factors that are consumer friendly but packed with medical-grade sensors capable of producing multiple vitals and biometric information. Another important development in RPM is the reliable and secure transport of data from a patient in the home to a remote application at the provider’s site. Managing data at the home involves data communication between the wearable sensor, an intermediate app such as on a mobile phone or router, and then into the cloud. Network reliability, power consumption, and user errors are common issues that need to be dealt with.
A complete RPM solution will need to include wearable sensors, edge processing in the home, reliable transportation of data to the cloud, and ultimately analytics and applications used for patient care and diagnostics. This is a tall order for any single solution provider to deal with and thus limits the pace of medical application innovation. However, a new class of medical sensors and data platforms such as VivaLNK’s IoT Medical Wearable Sensor Platform can reduce the complexities and barriers to RPM application development. By abstracting the device challenges in a remote environment, application development and integration becomes far simpler since it allows the solution provider to focus on the domain of their application instead of the device infrastructure.

CareTaker is a wireless patient vitals monitoring cuff that demonstrates this evolution in RPM. The form factor of the device and the solution’s ability to track multiple vital signs continuously are a vast improvement compared to traditional devices. When used in the home setting, the accuracy of data reported back to the provider and into the electronic health record (EHR) makes the data viable for use in treatment planning. It is not patient-reported data, but continuous, real-time, highly accurate information about the patient’s state that is sent to the cloud and connects the patient to the provider. This is where the true power of RPM lies.
The decentralization of care is inevitable. As a result, the growth rate of in-hospital devices and equipment will likely pale in comparison to the growth in RPM and devices that can be used in the home for health monitoring. RPM is here, but accelerating time to market is crucial. The advent of new sensors and the ability to access hardware and software in a single platform solves a variety of issues for manufacturers and OEMs so they can focus on the therapeutic expertise of their specific RPM application and more quickly get it to market.
VivaLNK
http://www.vivalnk.com

Explore the May 2019 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out
- Analysis, trends, and forecasts for the future of additive manufacturing
- BlueForge Alliance Webinar Series Part III: Integrate Nationally, Catalyze Locally
- Robot orders accelerate in Q3
- Pro Shrink TubeChiller makes shrink-fit tool holding safer, easier
- Revolutionizing biocompatibility: The role of amnion in next-generation medical devices
- #56 Lunch + Learn Podcast with Techman Robot + AMET Inc.





