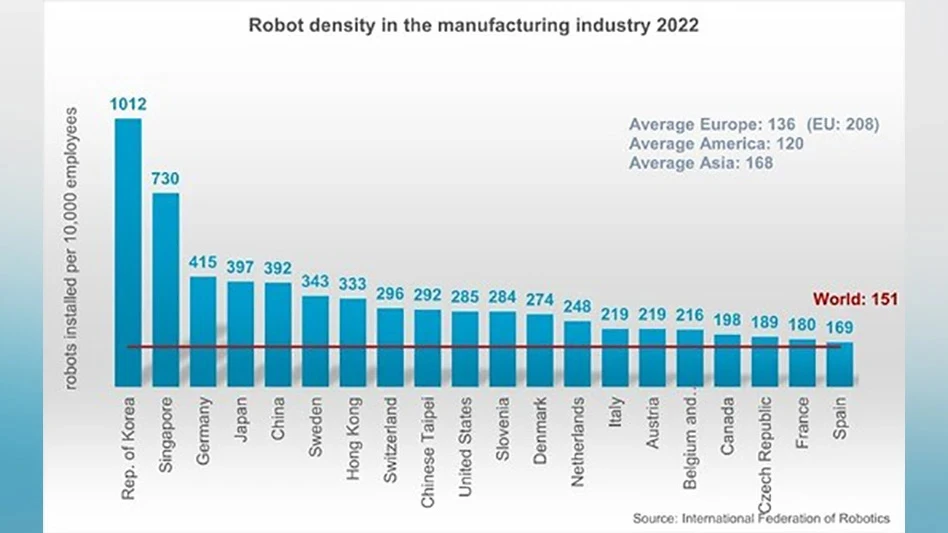
Anyone involved in manufacturing understands Murphy's Law. Regardless of how well a process is designed and implemented, anything that can go wrong will eventually go wrong. And even more troublesome, things get worse under pressure.
Speaking from my own experiences in manufacturing, I've seen people faced with the repetition of performing daily tasks get a little lax. They sometimes take things for granted and forget that even the smallest process deviation can have major consequences. And that's when old Murphy really pops up his head.
Yet, I truly believe all employees want to do a quality job.
From the front office to the shipping dock, employees, especially those involved in medical device manufacturing, must view quality as their number one responsibility. Regardless of how well a quality plan is written, it takes people to properly execute that plan day after day. This is why quality plans need to be written with total employee involvement in mind.
To prove my point, quality issues have recently appeared in some of the finest medical device manufacturing companies. Since the beginning of the year, one medical device manufacturer issued a warning to customers over potential hazards with volumetric infusion pumps used in hospitals to deliver intravenous fluids and medications to patients. The company warned that failure codes in the pumps could cause them to shut down while delivering fluids to patients. Another medical device company recalled approximately 14,000 defibrillators. The company said that there is a remote chance that the devices, available through prescription, may produce low-energy shock, shut down unexpectedly, or be susceptible to electromagnetic noise interference. Finally, taking steps to eliminate a potential problem, the FDA issued a warning letter to yet another medical device maker of catheters for faulty record-keeping. The FDA indicated it will not approve new devices to be manufactured at this plant until the company fixes the problems.
I mention these examples not to single out or embarrass any medical device company, but rather to emphasize the importance of quality and the absolute necessity of total employee involvement.
Now, the FDA is stepping up its enforcement of quality systems. In addition, medical manufacturers are also getting on board by designing and implementing compliance systems to standardize quality within its plants and divisions as well as standardize quality across its supply base in accordance with FDA regulations.
While there is no doubt that both the FDA and the medical device OEMs will accomplish these tasks, they must be mindful that successful implementation is a direct function of total employee involvement.
There must be an inducement to constantly keep employees involved with maintaining zero defects.
Then, and only then, will compliance systems and standard-operating-procedures generate the intended results.

Explore the June 2009 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Best of 2024: #5 Article – Accelerating medical device development with freeform injection molding
- Best of 2024: #5 News – Complexity, the enduring enemy of medical cybersecurity
- Best of 2024: #6 Article – Closing the global product information gap
- Best of 2024: #6 News – NUBURU enters medical device market with order Blueacre Technology
- Season's greetings
- Best of 2024: #7 Article – Synchronized machining processes for medtech
- Best of 2024: #7 News – 3D printing could revolutionize treatment for cataracts, other eye conditions
- Best of 2024: #8 Article – Perfecting the CMP process for surgical blades