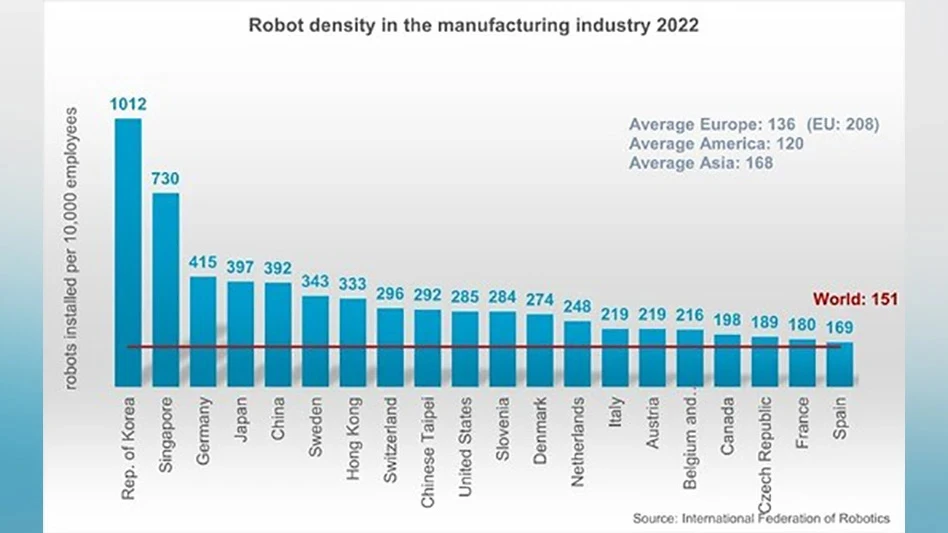
Danvers, Massachusetts – The U.S. Food and Drug Administration approved Abiomed's Impella 2.5 System, a miniature blood pump system intended to help certain patients maintain stable heart function and circulation during certain high-risk percutaneous coronary intervention (HRPCI) procedures, such as balloon angioplasty and stenting, which re-open coronary arteries that are narrowed or blocked due to severe coronary artery disease (CAD).
Coronary artery disease can lead to chest pain and heart attack and is the leading cause of death for both men and women in the U.S. It occurs when one or more of the major arteries on the surface of the heart become narrow or blocked, reducing blood flow that supplies the heart muscle with oxygen-rich blood.
The Impella 2.5 System is a heart pump intended for temporary use by patients with severe symptomatic CAD and diminished (but stable) heart function who are undergoing HRPCI but are not candidates for surgical coronary bypass treatment. In patients with diminished heart function, the heart pumps less blood than normal every time it beats, which is sometimes associated with CAD.
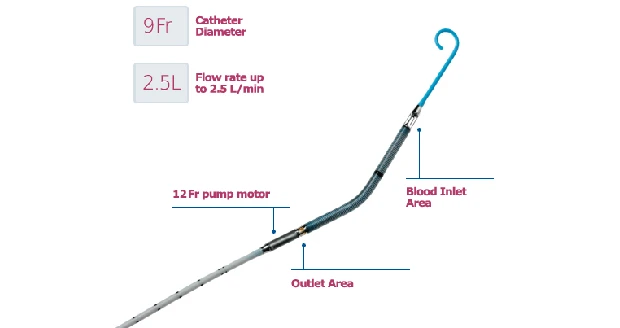
Throughout an HRPCI procedure, the Impella 2.5 System helps maintain stable heart function by drawing blood from the left lower chamber of the heart (left ventricle) and pumping it into the aorta, the main blood vessel leading away from the heart that supplies oxygen-rich blood to the body. Prior to starting a procedure, the interventional cardiologist places the Impella 2.5 using a catheter (a long narrow tube) with the pump loaded into the tip. The heart is accessed by inserting the catheter into one of the body’s large arteries, usually in the leg, and then guiding the tip of the catheter through the patient’s arteries and into the left ventricle. Once in place, an external controller and monitor turns the pump on and off, measures heart function, and allows health care providers to adjust the pump as necessary to maintain stable heart function and circulation during the procedure.
All patients undergoing HRPCI are at some risk for complications related to decreased heart function and lowered blood pressure during the procedure, but patients in need of treatment for extensive or critically located CAD who are already experiencing diminished heart function are at high risk. Unstable heart function that occurs during performance of an HRPCI procedure can result in serious complications or prevent completion of the procedure.
“Use of the Impella 2.5 System is intended to prevent episodes of unstable heart function, including unstable blood pressure and poor circulation, in patients who are at high risk for its occurrence,” said William Maisel, M.D., M.P.H., acting director of the Office of Device Evaluation in the FDA’s Center for Devices and Radiological Health.
The FDA reviewed data for the Impella 2.5 System in a premarket approval application, the agency’s pathway to evaluate a reasonable assurance of safety and effectiveness for class III medical devices. Data supporting the approval of the Impella 2.5 System included clinical data from the manufacturer’s PROTECT II clinical study with supporting information obtained from the USpella Registry, a multi-center, observational registry.
The overall data provided evidence that, for patients with severe CAD and diminished heart function, the temporary circulatory support provided by the Impella 2.5 System during a HRPCI procedure may allow a longer and more thorough procedure by preventing episodes of hemodynamic instability (e.g., poor circulation, low blood pressure) due to temporary abnormalities in heart function. Moreover, fewer later adverse events (e.g., need for repeat HRPCI procedures) may occur in patients undergoing HRCPI with the pump compared to patients undergoing HRPCI with an intra-aortic balloon pump (IABP). The Impella 2.5 System can be used as an alternative to the IABP without significantly increasing the safety risks of the HRPCI procedure.
Source: FDA
Latest from Today's Medical Developments
- Best of 2024: #5 Article – Accelerating medical device development with freeform injection molding
- Best of 2024: #5 News – Complexity, the enduring enemy of medical cybersecurity
- Best of 2024: #6 Article – Closing the global product information gap
- Best of 2024: #6 News – NUBURU enters medical device market with order Blueacre Technology
- Season's greetings
- Best of 2024: #7 Article – Synchronized machining processes for medtech
- Best of 2024: #7 News – 3D printing could revolutionize treatment for cataracts, other eye conditions
- Best of 2024: #8 Article – Perfecting the CMP process for surgical blades