Falls Church, Virginia – The Device Software Verification and Validation On-Demand Webinar will give attendees a clear understanding of FDA inspector expectations and how to meet them, with a specific focus on software that is as-product, in-product, process, and/or in overall manufacturing and operations.
It’s a crackdown for certain on device software verification and validation. The agency has hired a cadre of sophisticated enforcers, tough new inspectors trained in IT to recognize that software validation and documentation are the foundation for the safety of any device.
Veteran FDA consultant John Lincoln explains what the FDA expects now, and how to achieve it in a hurry.
In a 90-minute in-depth chalk talk, Lincoln demonstrates a preferred FDA 11-element documentation model in various applications; including ERP, in-device, as-device, process/equipment control, and cGMP data/Part 11 applications.
He addresses use of FDA guidances, GAMP 4/5, 21 CFR 11, and other applicable industry software validation models, coupled with the ISO 14971 / ICH Q9 Risk Management models and their real-world implementation.
Attendees will come away with an actionable understanding of:
- Developing and creating buy-in for a project validation plan
- Understanding what the FDA accepts as an appropriate documentation model
- Beyond just in-product software V&V – understanding FDA’s expectation for your testing software, ERP software and more
- When and how to use DQ, IQ, OQ, PQ or their equivalents
- How GAMP 4/5 requirements fit into your V&V planning and execution
- Detailing the FDA’s 11 key V&V documentation elements:
- Level of concern
- Hazard/risk analysis
- Software description
- SRS (software requirements specification)
- Architecture
- Design specification
- Traceability (matrix; paragraph numbering)
- Development
- V&V (verification/testing & validation: IQ, OQ, Part 11 test cases, if required, PQs)
- Revision history and release number
- Unresolved anomalies (Bugs)
- White Box and Black Box validations
Device Software Verification and Validation will give a clear understanding of FDA inspector expectations and how to meet them, with a specific focus on software that is as-product, in-product, process, and/or in overall manufacturing and operations. Why risk FDA sanctions, let alone multimillion-dollar liability lawsuits?
Who will benefit:
- Software development
- Programming, documentation
- Testing
- QA/RA
- R&D and engineering
- Manufacturing production and operations
FDAnews on-demand webinar
Dec. 9, 2014 – Any time at your convenience
Tuition
$487 per site -- includes webinar registration and audio CDs and transcripts
Easy ways to register
Online: http://www.fdanews.com/DeviceSoftwareVerificationOnDemand
Phone: 888.838.5578 or 703.538.7600
Source: FDAnews
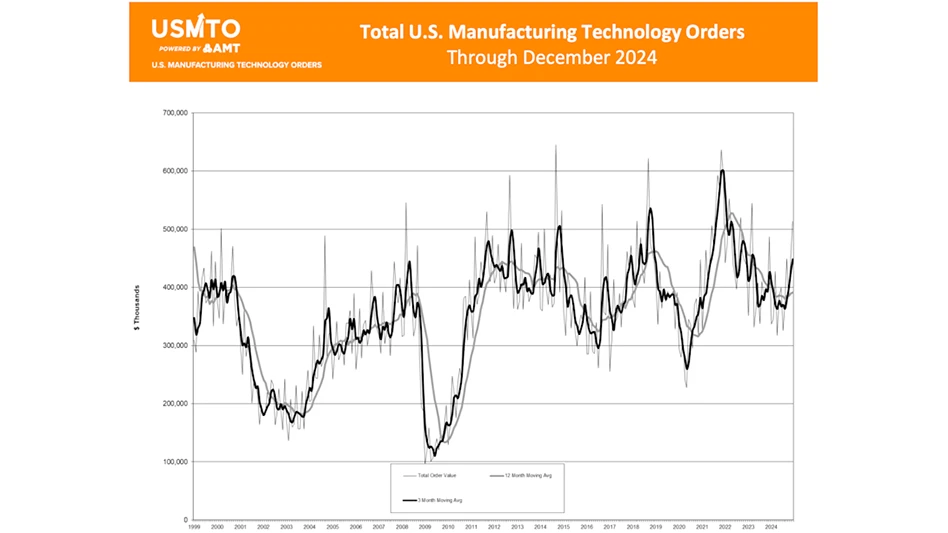
Latest from Today's Medical Developments
- Collaboration to advance pediatric medical technologies
- Guyson's TR-1000 Custom Vertical Blast System
- Intelligent data for the digital factory
- Edge Technologies' latest barfeeders
- Arterex expands again with acquisition of Adroit USA
- Teknor Apex material innovations for the healthcare industry
- Archetype appointed to secure EU Market approval for LumipenPro
- Learn from the experts in upcoming workholding roundtable