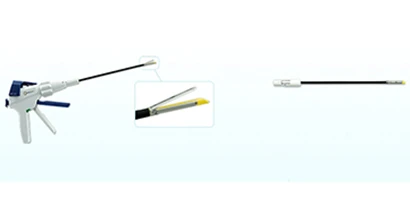
Boulder, Colorado – JustRight Surgical, a micro-laparoscopic medical device company devoted to miniaturizing surgical instrumentation, has received 510 (k) clearance from the U.S. Food and Drug Administration (FDA) for its JustRight 5mm Stapler. The stapler, the smallest classic stapler on the market, will be used by surgeons in pediatric and general surgery where access is limited and visibility is reduced. This is the third product from the company to win FDA clearance since its founding in 2010.
For the pediatric surgical community in particular this device represents a major step forward. Medical device options configured specifically for pediatric surgery have been limited. Since 1972, when pediatric surgery became a recognized subspecialty, surgeons have been asking companies to miniaturize instrumentation to better serve infants and children requiring surgery.
“The needs of our smallest patients are unique, yet the surgical tools available to surgeons for these cases are virtually non-existent,” said Dr. Steve Rothenberg, medical advisor and co-founder of JustRight Surgical. “In the electronics industry, miniaturization happens almost faster than we can absorb the changes. Meanwhile, the downsizing of surgical instrumentation has remained stagnant and we have made it our mission to change that.”
Features of the JustRight 5mm Stapler, include:
- 9 times smaller than existing stapling instruments
- Security of the gold standard B staple formation
- Flexibility in stapler placement with 5 mm shaft diameter
- Smooth and efficient stapling and cutting in one motion
- Optimal jaw length for maneuverability into small spaces
- Easy repositioning with 360° jaw rotation
- Developed and manufactured in the U.S.
“The absence of innovation in this market is primarily due to minimal financial incentives for large companies to develop these micro instruments,” said Russ Lindemann, president and CEO of JustRight Surgical. “As a result, pediatric surgeons are forced to use devices originally designed for adults. These instruments can be oversized, overpowered, and difficult to maneuver in small patients and tight spaces. We felt it was critical to answer the call for smaller devices and expand the options available.”
Source: JustRight Surgical
Latest from Today's Medical Developments
- Auxilium Biotechnologies prints medical devices on the International Space Station
- KYOCERA SGS Precision Tools’ APEX Application Expert
- North American robotics market holds steady in 2024 amid sectoral variability
- Evident’s DSX2000 digital microscope
- Ferrocene becomes first Rust toolchain to achieve IEC 62304 qualification
- Germany expects a major decline in production in 2025
- Learn what you need to comply with CMMC requirements
- VersaBuilt’s CNC automation possibilities