Drug delivery systems, such as those developed by Kindeva Drug Delivery, are the culmination of a series of carefully coordinated steps, where the active pharmaceutical ingredient (API) meets the patient. Preserving the sterility and efficacy of the product, and ultimately the safety of the patient, is paramount.
With a nearly 175-year history, Kindeva has played a role in bringing hundreds of drug products to fruition. Among the Minnesota-based company’s innovations is a proprietary solid microstructured transdermal system (sMTS) platform. The microneedle-based device is patient-friendly, making it ideal for self-administering abaloparatide, a biologic that stimulates bone formation for postmenopausal women at high risk for bone fractures due to osteoporosis.
Since abaloparatide is a biologic API, it can’t be terminally sterilized. Therefore, coating and packaging the abaloparatide-sMTS combination must take place entirely within an ISO Class 5 environment. In the lead-up to a new drug application (NDA) filing, Kindeva sought to reduce the product’s manufacturing cycle time. Only an automated system, capable of operating optimally within an aseptic isolator, could accomplish this.
The company turned to Keller Technology, a partner in robotic applications in biotech and pharmaceuticals. So, it was up to Keller to identify a key component: a robot that meets all the customer’s specifications.
Right robot for a sensitive environment
Stäubli Robotics was the choice for the sterile coating and packaging system as its Stericlean range of robots are designed specifically for these applications. Some of the features that enable Stericlean robots to operate in a good manufacturing practice (GMP) Grade A environment and maintain high performance under strict, aseptic conditions include:
- Fully enclosed structure, special seals minimize airborne particles
- Completely smooth robot surface, protected by a high-resistance coating, eliminates retention areas where antigens can proliferate
- Able to withstand harsh, vaporized hydrogen peroxide (VHP) decontamination processes
- Connections run through the base of the robot, safely outside the isolator
Keller had been successfully integrating Stäubli robots for years in various applications – including a Stericlean for a nearly identical sMTS application for Kindeva – so they knew it would deliver cleanliness, repeatability, and accuracy. A Stericlean 6-axis robot, exceeding Kindeva’s requirements with an ISO Class 4 rating, was selected for integration into the system.

High precision at commercial scale
The system Keller devised performs precision dip coating and primary packaging within an aseptic isolator. It begins when the sMTS devices are transferred into the isolator on trays, while the sterile liquid API is fed into a coating system, designed by Keller.
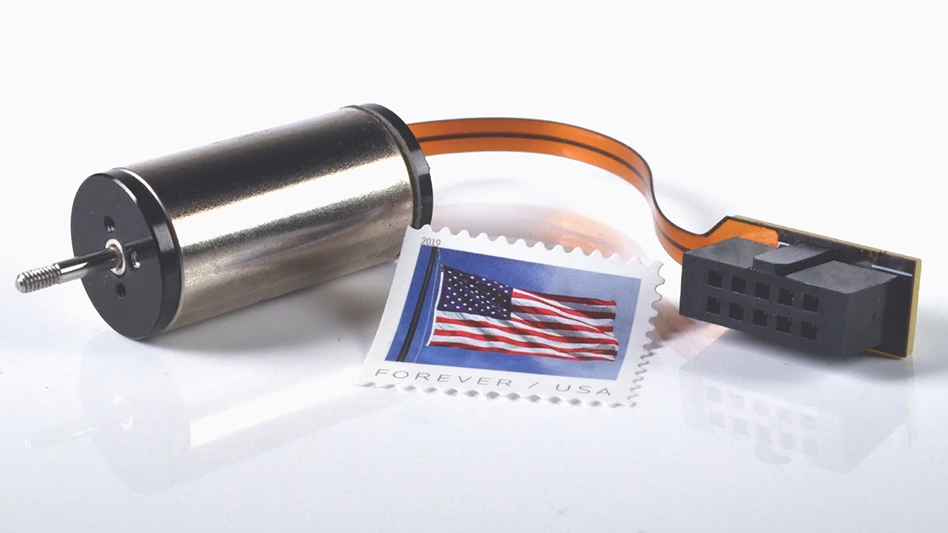
The Stericlean’s pinpoint accuracy is critical in the dip coating operation that follows. The dexterous robotic arm picks up the individual sMTS devices, each smaller than a postage stamp, and immerses them in the liquid API bath, loading the microneedles with the biologic API. The process is carefully calibrated to achieve repeatable, uniform coating on each unit.
The robot then lifts out the coated sMTS vertically, carefully places it back on the tray, and repeats the process. Once the tray is full, it’s transferred to a tray sealer, also custom-designed and built by Keller. Sealed trays are then transferred out of the isolator, completing the sterile primary packaging operation.
The isolator features a monitoring system to ensure no septic antigens are present. It also provides laminar airflow, so the entire body of air within the isolator is uniform in velocity and direction. All system components are designed to minimize airflow disruption, preventing disturbances such as eddies, voids, and shadows that could retain antigens.
Dip coating, reinvented
Keller’s automated system transformed a slow, difficult, and complex pharmaceutical manufacturing process into an inventive one, which includes the precision and repeatability of the Stäubli robot.
Crucially, the risks that are inherent to exposing a biologic API such as abaloparatide during dip coating are eliminated. Keller engineered and integrated its customized system into an aseptic isolator to maintain sterile conditions and shield the product from contamination. The specialized design of the Stericlean robot brings added assurance. This protects the operators, the product, and the patient.
While careful precautions are taken at every step of the production process, the automated system delivers the high speed and efficiency Kindeva needed to achieve its goal of reducing its abaloparatide-sMTS combination product’s manufacturing cycle time. Speed and efficiency gains through automation have enabled the company to scale up the product for commercial manufacture. The robot’s control software enhances traceability, optimizes process control, and has the potential to bring long-term benefits for years to come.

Explore the November December 2021 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Current economic and geopolitical realities demand decisive action
- Collaboration to advance pediatric medical technologies
- Guyson's TR-1000 Custom Vertical Blast System
- Intelligent data for the digital factory
- Edge Technologies' latest barfeeders
- Arterex expands again with acquisition of Adroit USA
- Teknor Apex material innovations for the healthcare industry
- Archetype appointed to secure EU Market approval for LumipenPro