Stewart & Stevenson
An enhanced version of the ApolloBVM designed by Rice University engineers has received Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) as an emergency resuscitator for use during the COVID-19 pandemic.
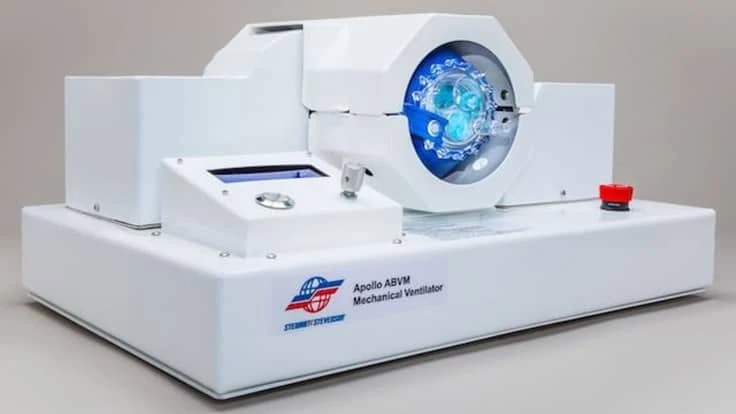
The device, as further developed by the Houston manufacturer Stewart & Stevenson Healthcare Technologies LLC, a subsidiary of Kirby Corporation, is designed to deliver air to the lungs of adult patients who require ventilation while they await the availability of a full ventilator.
The manufactured version, dubbed Apollo ABVM, is a sturdy and portable system the company believes can be rapidly deployed in emergencies during the EUA period.
Open-source plans for Rice’s ApolloBVM remain online and available to the maker community. The plans have been downloaded by nearly 3,000 registered participants in 115 countries.

“The COVID-19 pandemic pushed staff, students, and clinical partners to complete a novel design for the ApolloBVM in the weeks following the initial local cases,” says Maria Oden, a professor of bioengineering at Rice and director of the Oshman Engineering Design Kitchen (OEDK). “We are thrilled that the device has received FDA EUA.”
Development began in 2018 when a Houston emergency physician, Rohith Malya, brought his idea for a bag valve mask automation device to students at the OEDK at Rice’s Brown School of Engineering. The students designed and built a device that would squeeze a standard bag for hours on end, potentially saving lives.
A video produced by Rice as the students neared graduation in 2019 caught the attention of health professionals around the world last March, spurring a Rice team of staff engineers and one student, along with Malya, to revisit the “Take A Breather” device.
Working around the clock for weeks, the small team, alone in the OEDK during the initial pandemic lockdown, toughened the device and added safety features for use in a medical setting, a process continued by Stewart & Stevenson, which licensed ApolloBVM in April.
“The FDA authorization represents an important milestone achievement for the Apollo ABVM program,” says Joe Reniers, president of Kirby Distribution and Services. “We can now commence manufacturing and distribution of this low-cost device to the front lines, providing health care professionals with a sturdy and portable ventilation device for patients during the COVID-19 pandemic.”
Several manufacturing sites supported the effort, including manufacturing plants in Oklahoma City and Houston. “It is a testimony to the flexibility of our people and our manufacturing facilities that we are able to readily utilize operations to support COVID-19 related needs,” Reniers says.
Latest from Today's Medical Developments
- Best of 2024: #8 Article – Perfecting the CMP process for surgical blades
- Best of 2024: #8 News – Johnson & Johnson to acquire Shockwave Medical
- Best of 2024: #9 Article – Strategy Milling combines old and new for precision dental restorations
- Best of 2024: #9 News – Global robotics race
- Best of 2024: #10 Article – Designing medical devices for every user
- Best of 2024: #10 News – 4 predictions for 2024: AI set to supercharge robotic automation
- Children’s National, FDA collaborate to advance pediatric device regulatory tools
- LK Metrology’s eco-friendliness CMMs