Only one in ten medtech companies can easily share product registration data across their global regulatory teams.1 This creates a significant information gap across functions and regions for approximately 90%, which can lead to approval or renewal delays and lost revenue.
Regulatory affairs can fuel clinical and quality teams with key product information, help speed time to market, and be a catalyst for innovation. But the truth is most medtechs aren’t seeing these benefits, slowed down by inefficient administrative tasks.
Instead, many rely on disconnected legacy systems requiring regulatory teams to manually pull reports and documents to share with in-country and global teams. Not having one source of information is a significant challenge that will be especially critical as EU Medical Device Regulation (MDR) and In Vitro Diagnostics Regulation (IVDR) expand submission requirements. Evaluating processes and technology can help medtechs drive real-time access to data for proactive decision-making.
Legacy ways of working are holding the industry back

More than half of medtech regulatory professionals (56%) say their in-country regulatory affairs (RA) teams cannot access a single document source to support global submissions (Figure 1). This increases vulnerability to errors as teams turn to manual processes and communication methods.
More than one third (35%) rely on status meetings to share regulatory plans across functions. But what happens if an individual is out sick, is pulled into another meeting, or leaves the company? Critical information will remain with the individual (Figure 2). Manual errors, inefficient communication methods, and siloed or unavailable data deter innovation. To keep submissions moving forward, individuals must communicate vital information manually, reducing visibility and data quality. These legacy processes also lead to teams not seeing eye to eye.
Three-quarters (75%) say content, such as intended use or device descriptions, is sometimes misaligned across functions. With the variance in information, organizations face an increased risk of inaccurate or incomplete regulatory submissions.
Using legacy processes and disconnected systems can place RA teams in stressful audit situations. Many find it difficult to locate objective substantiation evidence in the event of a product claim audit by a regulatory authority. As a preventative measure, RA teams are often left to shoulder the burden of gathering, maintaining, and locally storing information required for audits.
Without a standardized and consistent way to share documents and data globally, organizations can’t ensure the reliability or accuracy of product information across markets. The increased compliance risk of relying on legacy processes can delay the delivery of devices and diagnostics to patients.
Rethinking regulatory affairs for greater speed and transparency
It’s time to think differently about RA to drive visibility, speed, and quality. Companies should prioritize establishing centralized regulatory data and documents, especially as medtech strives to accelerate approvals and speed new market entry. To modernize RA operations, start by evaluating processes and systems to identify the best path forward.
Create a brain trust of stakeholders to inform decision-making
No one can explain a broken process better than the people doing the work daily. Talking to stakeholders across regions can help identify critical areas for improvement or modernization.
Dig into processes for insights into problem areas
A challenge RA teams face can feel more real when you see it in action. Mapping out processes will highlight where regulatory teams are getting stuck. Once identified, consider where to save steps, time, or cost and how that can help regulatory teams focus on value-added activities.
Technology can power an entirely new way of working
Modern solutions can simplify regulatory processes while delivering visibility. Hearing from stakeholders and mapping out key processes will inform tools to best address existing challenges around data access and accuracy.
An advanced regulatory information management (RIM) system for new product submission planning can accelerate the process. By leveraging a RIM solution, organizations can empower RA teams and help them effectively manage product and registration information, submission dossiers, and marketing plans. First, establish clear goals for implementing a RIM system and what the end state will look like.
“It’s important to understand organizational priorities before embarking on a system change. At Exact Sciences, one of our long-term goals is to shift from manual processes toward digital technologies,” says Jaya Vaishnav, senior IT project manager at Exact Sciences.2
A RIM solution represents a vital step toward bringing together data and documents, eliminating the need for individuals to take on the burden of obtaining and sharing information. Keeping the focus on end goals pays off.
Accelerating regulatory transformation
As the regulatory landscape continues to evolve, the importance of regulatory affairs has never been greater. Establishing a single source of truth reduces time spent gathering information and allows teams to focus on assessing the impact of regulations on product portfolios instead of sending status emails. By closing the global product information gap with streamlined processes and advanced systems, RA can focus on driving innovation and helping products reach new markets faster.
1 Veeva Systems, 2023 Medtech Regulatory Benchmark Report, 2023
2 Veeva MedTech, Baxter and Exact Sciences: Lessons Learned from RIM Transformation, 2023
Veeva MedTech
https://www.veeva.com/medtech

Explore the March 2024 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Engineering empathy: Rice University students develop accessible Parkinson's device
- Revolutionizing precision: Tsugami BW329Z Swiss-type lathe
- #59 - Manufacturing Matters: Additive manufacturing trends, innovations
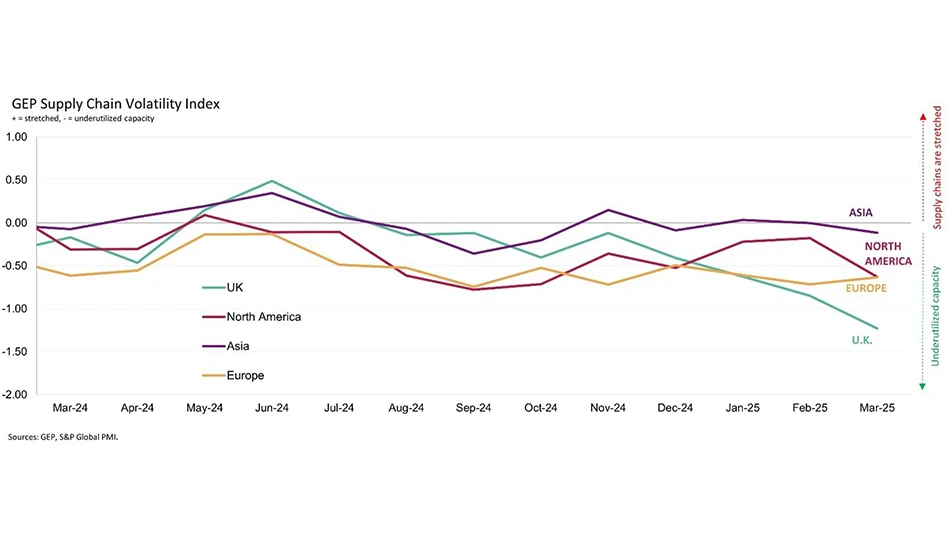
- Unlocking GenAI's potential: Insights from the Supply Chain Horizons 2025 report
- Celebrating 75 years of innovation at Jorgensen Conveyor and Filtration Solutions
- Free webinar to offer expert advice on optimizing machining operations
- How collaboration between companies can elevate manufacturing
- AI meets innovation: Cambridge's device transforms heart screening