According to the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH), heart failure affects 5 million people in the United States, with 550,000 new cases each year.
Addressing these health issues are technological advances that continue to show promise as permanent replacements for defective hearts. With all these developments, the heartassist device market could reach between $8 billion and $10 billion worldwide within the next few years.
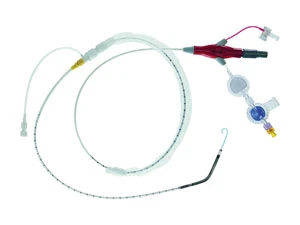
Once such advance is Abiomed Inc.'s Impella 2.5 circulatory assist device, which is for use in high-risk percutaneous coronary intervention (PCI) procedures. Results from the PROTECT 1 trial that evaluated the feasibility and effectiveness of the Impella 2.5 were published in a recent issue of the Journal of American College of Cardiology, and conclude that, "The Impella 2.5 system is safe, easy-to-use, and provides excellent hemodynamic support during high-risk PCI." SMALLEST PUMP The Impella 2.5 - the world's smallest heart pump - is only slightly larger in diameter than a drinking straw. With rapid and simple insertion, the minimally- invasive, catheter-based device provides circulatory support to the heart, and is intended to support the heart in situations where it cannot optimally function on its own.
One of the main advantages of the Impella 2.5 is that it may eliminate the need for an open-chest incision and placement of the patient on heart/lung bypass. Instead, the Impella 2.5 is inserted into the left ventricle in a catheterization lab (cath lab) via a standard catheterization procedure through the femoral artery, into the ascending aorta, across the valve, and into the left ventricle within a few minutes. The tip of the catheter contains a "pigtail" that crosses the aortic valve and rests in the left ventricle. Designed to actively unload the left ventricle by pumping up to 2.5L of blood per minute, the Impella 2.5 increases cardiac output, reduces myocardial workload and oxygen consumption, and increases coronary and end-organ perfusion.
Once the Impella 2.5 is secured, the device, which has a shielded propellerlike piece, pulls the blood in, speeding up the flow of blood and making it easier for the heart to do its job. Blood flow is improved to vital organs and to the heart. As the Impella 2.5 functions by speeding up the flow of the blood, the regular balloon angioplasty and/or stent procedure can be completed with the Impella 2.5 being removed after the procedure.
THE DESIGN
Mechanical, electrical and polymer components comprise the design of the Impella 2.5:
- Impella 2.5 catheter
- Impella console
- Impella power supply and power supply cable
- Braun Vista basic infusion pump
- Impella Combitrans-Monitoring set
- Pressure transducer cable
- Connector cable
- Testplug pump
- Introducer kit
- 0.014/260cm guidewire
- Braun Vista pump set
20 Dextrose in water The Impella 2.5 catheter itself is an intravascular micro-axial blood pump comprised of 14 miniature components.
Beginning at the tip of the Impella is the 6F pigtail, attached to the cannula at the distal end of the inlet area, which assists with stabilizing the catheter in the correct position within the left ventricle.
Following is the cannula, which is 12F in diameter and has a spiral-shaped reinforced body shaped in a 45° angle.
The inlet area - where the blood enters the cannula - is located at the distal tip while the proximal end of the cannula is attached to the outlet area where the blood exits the cannula.
Next is the micro-axial blood pump, which is located between the cannula and the catheter - where the motor housing is 12F in diameter and consists of a completely encapsulated motor. Following is the open pressure area, located between the motor housing and the distal end of the catheter.
A pressure lumen transitions from the open pressure area and runs the length of the catheter into the plug. A pressure sensor is located in the plug and enables the pressure to be displayed on the Impella Console.
Next is the 9F catheter shaft, which is located between the motor housing and the plug. The lumen of the catheter shaft contains a purge lumen, a pressure measurement lumen, and a pump monitoring cable. Important with the catheter shaft is the repositioning unit, which consists of an introducer - graduated from 9F to 12F - located on the catheter shaft and an anti-contamination sleeve with an anchoring ring.
The introducer - with hemostatic valve - allows for repositioning of the catheter.
The anchoring ring of the anti-contamination sleeve is used for securing the catheter sheath to the introducer.
The plug, which is the section of the catheter that connects to the Impella Console through a connector cable, is located at the proximal end of the catheter. An infusion filter, pressure reservoir, and check valve are located off of the clear sidearm from the plug.
The filter prevents bacterial contamination and prevents air from entering the catheter. The check valve ensures that purge fluid does not flow in the reverse direction when a purge solution is exchanged. The pressure reservoir includes a flexible rubber diaphragm that provides additional filling volume by means of an expansion chamber during purge solution change.
An external motor, the Impella Console, controls the micro-axial blood pump and monitors the system, keeping blood flowing in the weakened heart. The Console weighs 7 lb and operates on an internal lithium-ion (Li-Ion) battery for at least 60 minutes when fully-charged. The Impella Power Supply provides and charges the battery within the Impella Console.
A multi-voltage power source for use with voltages within a range of 100V to 240V, at line frequencies of 50Hz and 60Hz, the integrated charging circuit can fully charge the Impella Console Li-Ion batteries in about 10 hours.
Rounding out the system is the Braun Vista basic Infusion Pump that delivers purge fluid through the catheter to the micro-axial blood pump preventing blood from entering the motor.
In June 2008, the Impella 2.5 received 501(k) clearance from the FDA for partial circulatory support for periods up to six hours, is approved in more than 40 countries including earning the CE mark, and has been used to treat more than 1,700 patients outside the United States.

Explore the June 2009 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Best of 2024: #5 Article – Accelerating medical device development with freeform injection molding
- Best of 2024: #5 News – Complexity, the enduring enemy of medical cybersecurity
- Best of 2024: #6 Article – Closing the global product information gap
- Best of 2024: #6 News – NUBURU enters medical device market with order Blueacre Technology
- Season's greetings
- Best of 2024: #7 Article – Synchronized machining processes for medtech
- Best of 2024: #7 News – 3D printing could revolutionize treatment for cataracts, other eye conditions
- Best of 2024: #8 Article – Perfecting the CMP process for surgical blades