Medical device designers’ goals have always been enhancing and enriching device functionality while reducing size and cost. This is particularly true as modern devices start acting like complex miniaturized computers for the body, interfaced with microelectronic sensors and actuators. In response, medical device engineering teams – designers and manufacturers – are using advanced semiconductor assembly techniques, tools, materials, and methods, which often employ specialized adhesives.
Medical adhesives are used during assembly and subassembly to adjoin various microelectronic, structural, and functional components within tiny devices, but they should be selected at the design stage. Medical device designers need to learn more about adhesives and partner with manufacturers who have expertise sourcing, selecting, and working with epoxies to make optimal choices. Early adhesive decisions affect the development, manufacturing, cost, performance, and reliability of the device. The days of simply sticking things together with glue are long gone. Today’s complex devices require careful selection of medical grade adhesives based on their fundamental properties and how they can optimally join together various materials and geometries.
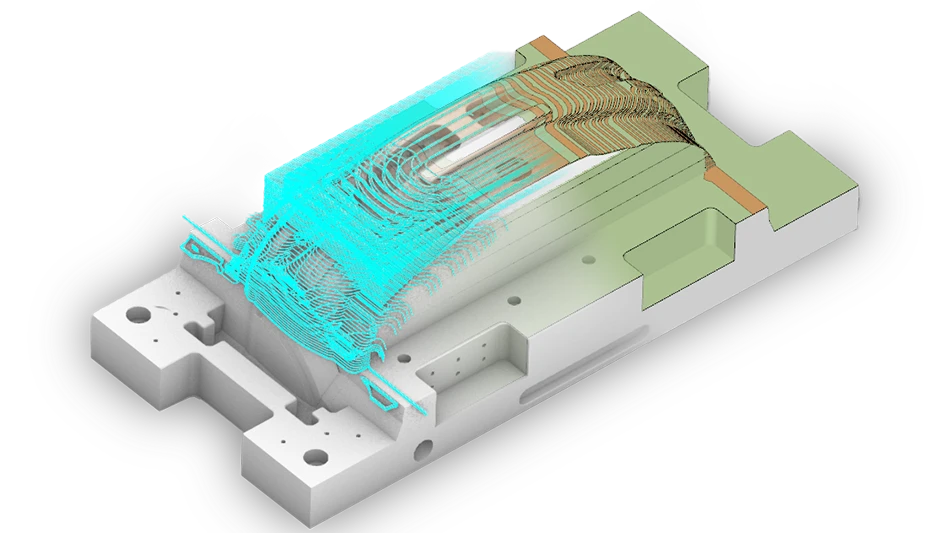
Structure of a modern, miniaturized medical device
Today’s medical devices are made using flat carrier substrate boards, such as an organic laminate, flexible or rigid flex circuit, or alternative materials (e.g., ceramic or glass). The substrate provides electrical interconnections between components and signal/power connections into and out of the miniaturized device (via a battery or wirelessly through an inductive coupling). The substrate serves as the mechanical backbone for the device.
Other components of the device include onboard computing chips, passive components, supporting active components (such as discrete amplifiers and power converters), specialized actuators that deliver energy, sensors that detect signals, other supporting parts (such as mechanical or optical elements), and an external housing.
Adhesives attach the components and form a structural interface between them and the substrate. Adhesives can save space, provide specialized capabilities (such as optical or thermal transfers), and are often the only viable adjoining method available. They can be rapidly dispensed by automated systems with high precision, which simplifies the medical device manufacturing process and decreases the cost of making the device at high volumes.
Cured adhesives

Cured adhesives have various properties that must align with a device’s final requirements. Common classes of cured adhesives are:
- Electrically conductive adhesives – Commonly used in place of solder to connect microelectronic components within a circuit.
- Electrically insulating adhesives – Used to physically connect devices without shorting out connections.
- Thermally conductive adhesives – Transfer heat effectively between adjoined surfaces; good for use between power-hungry amplifiers, light-emitting diodes, and heatsinks. Some medical devices require temperature-control elements bonded to hardware/temperature sensors using thermally conductive adhesives.
- Optical adhesives – Two categories are used to form optical chains: highly transparent materials mechanically bonding elements and highly light-blocking materials absorbing scattered or stray light. Some of these materials vary transmission or blocking as a function of wavelength (color).
- Highly rigid adhesives – They provide structural integrity to the device system. They’re often filled with glass or other micro-particles to increase stiffness and compression resistance.
- Flexible adhesives (often silicones) – They’re gaining traction within wearable medical devices, especially when combined with very thin, flexible semiconductor dies, for flexible circuit board substrates.
Uncured adhesives
Uncured adhesives have equally varied properties. With uncured epoxies, it’s important to understand where the glue should or shouldn’t go before it’s cured. Knowing this can determine how and when it should be dispensed within the manufacturing process. When using uncured adhesives:
- Ensure clearance for dispensing equipment to access appropriate areas without crashing into other components.
- Predict the risks for downstream yield loss during manufacturing, caused by epoxy squeeze-out beyond a component, which would block other parts from their appropriate locations later in assembly. Squeeze-out can also compromise wire bond pads, preventing electrical connection and impacting long-term reliability of the contaminated joints.
- Know if the epoxy can withstand subsequent manufacturing assembly steps.
- Mitigate squeeze-out at the design stage by designing appropriate keep outs between epoxy-bonded components.
- Select appropriate adhesives for the desired application knowing viscosity is a critical parameter. For example, thixotropic adhesives have decreased viscosity when stressed, shaken, or agitated but become much thicker when the stress is no longer present. These epoxies can be dispensed with very fine control, but then will stay in place after dispensing is complete. These epoxies are a mainstay for microelectronic applications.
- Know the working life of the medical adhesive before it starts to harden. Pot life is defined as the time required for the initial viscosity of the uncured adhesive to double, indicating curing initiation. Carefully consider the manufacturing process and batch size with respect to pot life to avoid issues with waste or limitations on volume opportunities in the future.
- Respect curing conditions. Elevated temperatures for curing adhesives are often required. Can the parts in the device tolerate the required temperature? Sometimes UV-curable adhesives are an alternative, but only if the surfaces inside the device are transparent.
Speaking the language of medical epoxies
- Die attach film (DAF) is a laminated adhesive film that is bonded to wafers or other flat sheets prior to die singulation (sawing). DAF has a major advantage – it reduces manufacturing costs by improving workflow by eliminating the need for dispense equipment. Squeeze-out is also mitigated as the DAF outline, constrained to the sawn die and bondline thickness, is extremely uniform. DAF films are available with many physical properties (conductive, insulating, thermally conductive of different modulus) and thicknesses.
- Bondline thickness measured between adjoined surfaces is often critical. Typically, thinner is always better for the structure. Thermal-transfer applications, for example, almost always benefit from very thin bondlines; minimizing bondline thickness between surfaces linearly diminishes the impact of an adhesive’s lower thermal conductivity than either mated surface, improving thermal transfer across the joint. However, it’s critical in thermal applications to eliminate voids within the adhesive, as air is a tremendous insulator. Hot spots within a thermally sensitive device can deleteriously impact its performance and reliability.
- Bondline parallelism is another consideration between bonded surfaces. Some materials are engineered to be self-leveling, where the bondline is defined by particles within the adhesive.
System requirements impact adhesive choice

Finally, it’s important to carefully consider certain conditions affecting the ability to manufacture a device at high yield and ensure desired performance, reliability, and durability in the field.
- Conditions – Ship, store, and use conditions the device is exposed to commonly impacting its adhesives’ mechanical and special properties. These conditions include the absolute rate of change for temperature, humidity, and UV exposure.
- Biocompatibility – Special epoxies qualified to ISO biocompatibility standards should be selected during the design stage if they’re vital to the finished device.
- Chemical resistance – Choose materials that’ll withstand the product’s cleaning requirements and typical use/misuse cases.
- Autoclavability – If required for the device, is the adhesive qualified to be autoclavable?
- Shock/vibration – Adhesive encapsulation can make a device more rugged with proper design.
- Supply chain considerations – Exotic adhesives can be hard to source and have short shelf lives, increasing lead time and cost. Think about using a more common material.
Expertly choose and use adhesives that hold together all the delicate components of a modern medical device. While typically an afterthought, adhesive considerations should now be top-of-mind. Adhesive knowledge at the design stage and working with manufacturing partners who can educate and inform throughout development and assembly are key to achieving quality, efficiency, and scalability of your medical device.
Promex
https://promex-ind.com

Explore the March 2024 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- Unlocking GenAI's potential: Insights from the Supply Chain Horizons 2025 report
- Celebrating 75 years of innovation at Jorgensen Conveyor and Filtration Solutions
- Free webinar to offer expert advice on optimizing machining operations
- How collaboration between companies can elevate manufacturing
- AI meets innovation: Cambridge's device transforms heart screening
- Mazak and Premier Engineering team up for greater agility in Florida
- Struggling with inventory or supply chain pressures? Find answers in our free webinar
- Free webinar: Advanced manufacturing solutions to support the Navy