Photo ©ALPHASPIRIT | Adobe Stock
Medical device manufacturers must establish and follow quality systems in accordance with U.S. Food and Drug Administration (FDA) current good manufacturing practices (CGMP) to ensure that devices are safe and function as intended. The quality system regulation (QSR) doesn’t prescribe how to produce a specific device, but states manufacturers “should use good judgment when developing their quality system and apply those sections of the QSR that apply to their products and operations.”

It’s the manufacturer’s responsibility to establish the appropriate methods and procedures to design, produce, distribute, and deliver devices meeting these requirements, which involves creating or selecting a quality management system (QMS).
Many factors can steer QMS solution decision-making. Every stakeholder should consider the entire product release process to avoid myopic decisions. Take into account siloed information and disconnected teams, so everyone has full context throughout the new product development and introduction (NPDI) process.
To help ensure broader business objectives can be met, consider these five key questions when assessing your quality needs.
1. Does the QMS manage the entire product record?
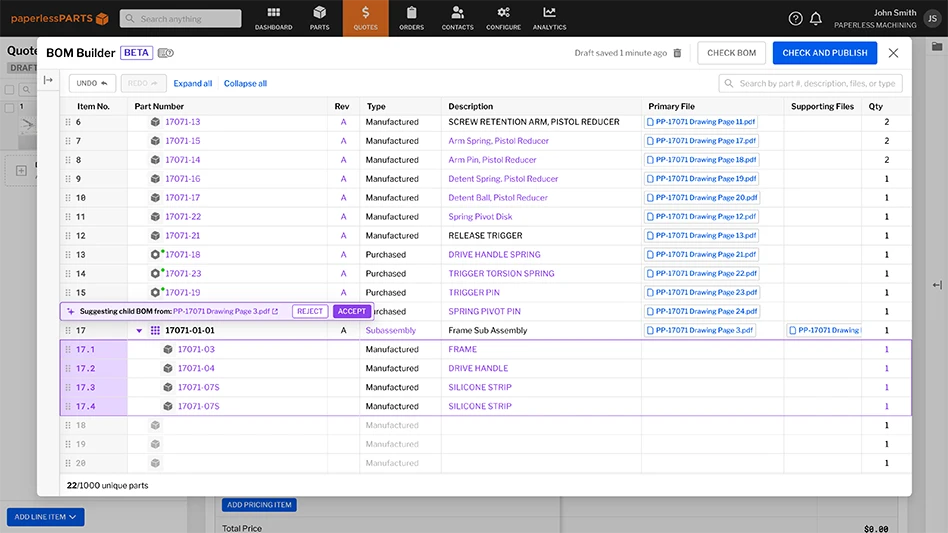
Product records for complex medical devices include documents, components, assemblies, and the entire product record – organized in a relational, multitiered bill of materials (BOM). The complete BOM needs to aggregate parts and documents, track them by controlled revisions, and link every part or document to associated quality records and processes.
Traditional document-centric QMS solutions have significant gaps in managing the entire product record and BOM comprised of vast amounts of mechanical, electrical, and software components. They focus on collections of documents. However, product-centric QMS solutions are built on the product record to provide a relational, hierarchical BOM with every component, drawing, specification, manufacturing work instruction, standard operating procedure (SOP), and training record linked together for maximum visibility and transparency. These BOM structures are organized in the same manner as upstream design systems (CAD) and downstream manufacturing systems (ERP) to enable seamless passing of controlled BOMs from design to QMS to manufacturing. This product-centric approach gives engineering, quality, operations, and supply chain teams a complete picture of all records and related processes to ensure devices can be built as designed, while complying with FDA, ISO, or other regulations.
2. Does QMS elevate the corrective and preventive action (CAPA) process?
When any issue or problem occurs, having a way to quickly identify the root cause and correct the problem is critical. Determining the cause requires thorough documentation of the issue and the ability to efficiently drill into the product design record and/or manufacturing process. Only after the what/when/why/where/how questions are answered can the corrective action be identified and executed.
To design, test, and deliver high-functioning products, a quality system should improve visibility and traceability between the product design and the CAPA process. A product-centric QMS approach is the best way to manage all related documents in context with both product and quality processes. Having a single connected system simplifies investigating and resolving quality issues.
3 . Does QMS empower disparate teams to collaborate effectively?
Proactive, real-time notification speeds collaboration. And providing historical audit trails for design, test, and approval processes is critical to prevent cross-functional team blind spots. If an organization’s QMS solution can’t easily provide access to every impacted player at every stage of the NPDI process, product development and quality issue resolution delays are more likely to occur. Failing to prevent or identify issues early in the development process or after the product has been shipped to customers can prevent a medical device company from continuing to sell into a given market.
Web-based cloud solutions such as product-centric QMS connect teams 24/7 and speed communication, eliminate design errors, and spur rapid innovation. This multidisciplinary collaboration makes it easy for every team to review information and respond immediately by reviewing product designs, associated documentation, and quality records in a single system to drive continuous improvement, increase product reliability, and enhance manufacturability.
4. Does QMS support quality processes before and after products are shipped?
The importance of quality management doesn’t diminish after initial compliance to regulations or after products are delivered to market. Medical device companies must have a fully traceable quality and design process that spans many years and many patients. Since quality issues can arise before or after devices have gone to market, having a QMS that provides a historical audit trail for everyone involved in designing, testing, delivering, and sustaining is key. This ensures the medical device manufacturer can trace the design and quality records to determine design or manufacturing issues throughout the entire product life cycle.
5. How difficult is it to validate the QMS software?
The FDA requires medical device manufacturers to validate software used in the design or delivery of devices. However, validation is often considered an overhead cost. Meeting the FDA’s validation regulations for traditional on-premises or document-centric QMS environments requires significant internal resources and an understanding of the software capabilities, and specific use cases.
Product-centric QMS solutions with multi-tenant cloud architecture eliminate traditional obstacles and alleviate resource challenges, simplifying a large portion of the validation process. Your QMS vendor should design and support validation requirements to reduce the impact to internal teams. Be sure to ask whether the QMS has standard validation services and documentation to remove the heavy lifting for installation qualification (IQ) and operational qualification (OQ) – leaving mostly customer-specific performance qualification (PQ) requirements to be executed by your internal team. Multi-tenant cloud solutions offer greater scalability of IQ and OQ validation support since they leverage the same infrastructure across all customers.
Designing, producing, and supporting complex products is challenging with distributed teams and supply chains. For medical device manufacturers, the ability to ship quality devices is more difficult due to stringent FDA and ISO regulatory compliance. To innovate and ensure compliance and positive patient health outcomes, it’s essential to have a product-centric QMS approach. These five questions will help identify whether a QMS solution can support the delivery of high-quality devices while maintaining regulatory compliance and providing better traceability throughout the entire product lifecycle.

Explore the March 2020 Issue
Check out more from this issue and find your next story to read.
Latest from Today's Medical Developments
- North America's supply chains face sharp decline due to tariffs
- Experience precision: GF Machining Solutions' CUT F Series wire EDM
- Mastering high-temp alloys with Kennametal Inc.
- Integer expands operations in Salem, creating 83 jobs
- Siemens unveils new Teamcenter X: Revolutionizing SaaS PLM for all manufacturers
- 3 Questions with an Expert with Allied Machine & Engineering
- Supply Chain Power – A strategic program for executives
- Sunnen Products' PGE-6000 gage