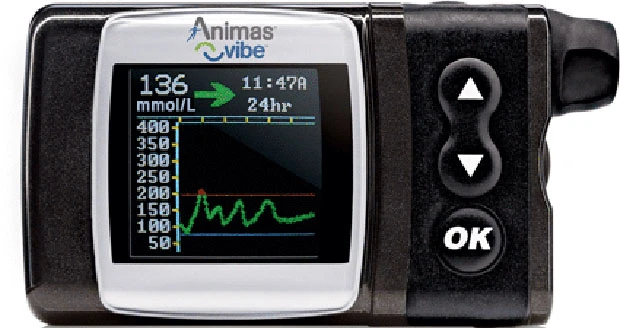
The new product from Animas, a division of Johnson and Johnson, is the first to incorporate the Dexcom G4 Platinum CGM technology, which will assist the management of people with insulin-requiring diabetes of 18 years and above.
The CGM technology samples interstitial fluid every 5 minutes, providing a trend of blood sugar levels and then allowing users to read data from the pump screen itself.
Users of the Animas Vibe can have basal insulin delivery pre-programmed, enabling precise insulin dosages to be delivered depending on blood glucose readings. A push of a button can pump in bolus insulin when appropriate.
The Animas Vibe insulin pump is waterproof for up to 12ft for 23 hours. The transmitter of the Dexcom CGM is also water resistant up to eight feet for 24 hours.
Other features of the Animas Vibe include customized dosing, which allows for up to 12 personalized settings of insulin-to-carbs ratio and insulin sensitivity, as well as customizable alarms to indicate high or low blood glucose levels.
Source: Animas Corp.
Latest from Today's Medical Developments
- Arcline to sell Medical Manufacturing Technologies to Perimeter Solutions
- Decline in German machine tool orders bottoming out
- Analysis, trends, and forecasts for the future of additive manufacturing
- BlueForge Alliance Webinar Series Part III: Integrate Nationally, Catalyze Locally
- Robot orders accelerate in Q3
- Pro Shrink TubeChiller makes shrink-fit tool holding safer, easier
- Revolutionizing biocompatibility: The role of amnion in next-generation medical devices
- #56 Lunch + Learn Podcast with Techman Robot + AMET Inc.





